Product Recalls
Dannon YoCrunch
Dannon has issued a voluntary recall on several batches of Dannon YoCrunch® Yogurt with the lot numbers listed below due to a potential presence of plastic pieces in the dome that contains the toppings.
Consumers with questions may contact YoCrunch® Consumer Care Line at 1-877-344-4886.
PRODUCT DISCRIPTION
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
4/4 Oz |
DANNON YOCRNCH VAN OREOS |
ALL |
ALL |
00046675013501 |
|
4/4 Oz |
DANNON YOCRNCH VAN MMS |
ALL |
ALL |
00046675013518 |
|
4/4 Oz |
DANNON YOCRNCH STRWBRY MMS |
ALL |
ALL |
00046675013129 |
Published July 14, 2025
ZICAM COLD REMEDY NASAL SWABS
Church & Dwight Co., INC. has issued a voluntary recall on all batches of ZICAM™ COLD REMEDY NASAL SWABS due to a potential contamination.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
20ct |
|
All Lot Codes |
All Dates |
732216301205 |
This voluntary recall is the result of a recall potential contamination.
Consumers who want to verify if their product is affected by the issue may do so at www.churchdwightrecall.com.
THAT’S SMART VEGETABLE OIL
That’s Smart Vegetable Oil W/ Blue Lid has some product reported as mislabeled, the 40 oz bottle with a blue lid could be labeled as Canola oil when it is Vegetable oil which contains soybean oil. This could result in an allergic reaction due to the soybean oil, which is a major allergen.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
40oz |
That’s Smart Vegetable Oil with the blue lid |
Product with the blue lid should say Vegetable Oil on the front and list Soybean Oil as the ingredient on the back. If the label and the ingredient listed is Canola oil the product is mislabeled and could cause allergic reaction |
N/A |
193476006208 |
This voluntary recall is the result of mislabeled That’s Smart Vegetable Oil – W/Blue Lid, the product could be labeled as canola oil when it is vegetable oil which contains soy which is a major allergen. This could cause an allergic reaction due to soy as an ingredient
Consumers who have an allergy to SOY should not consume any remaining product and are advised to return the product to the place of purchase for a full refund. If you are not allergic to SOY the products are safe for consumption.
LC Lemon Garlic Shrimp Bowls
Nestle USA has issued a voluntary recall of Lean Cuisine Lemon Garlic Shrimp Bowls with the lot numbers listed below due to possible wood- like substance in product.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
10 OZ |
Lemon Garlic Shrimp Bowl |
4214595511 |
SEPT2025 |
13800333131 |
This voluntary recall is the result of Lean Cuisine Lemon Garlic Shrimp Bowls due to possible wood- like substance in product
Consumers are advised not to consume product are to return the product to the place of purchase for a full refund.
Consumers with questions may contact - Nestlé USA at (800) 681-1676 Monday-Friday from 9 a.m.-6 p.m. EST.
Gerber Sooth N Chew Teething Sticks
Gerber has issued a voluntary recall on all batches of Gerber Soothe N Chew Teething Sticks listed below due to a Potential Choking Hazard.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
3.2 OZ |
Strawberry Apple |
All lot codes |
All |
015000046187 |
|
3.2 OZ |
Banana |
All lot codes |
All |
015000046088 |
This voluntary recall is the result of a recall due to a Potential Choking Hazard.
Please discontinue use immediately and return any remaining product to the place of purchase for a full refund.
Consumers with questions may contact Gerber 24/7 via their Gerber Helpline at 1 - 800 - 443 - 7237
MadeGood Granola Bars
Riverside Natural Foods Inc. is voluntarily recalling MadeGood Granola Bars, due to the potential presence of a piece of metal in the product. Consumption of this product may result in a safety hazard.

Riverside Natural Foods Inc. is voluntarily recalling MadeGood Granola Bars, due to the potential presence of a piece of metal in the product. Consumption of this product may result in a safety hazard.
This voluntary recall is the result of a recall to possible metal in product.
Consumers can contact MadeGood with questions at 855-215-5695 between 8am and 5pm EST.
American Snuff - Grizzly
American Snuff Company has issued a voluntary recall on Grizzly LC Straight snuff products due to the potential adulteration. Please note that characters represented as “x” in the codes below will vary and are not relevant to the identification of affected product.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
1.2 oz |
Grizzly LC Straight |
GxxQxQK4, GxxRxQK4, GxxSxQK4, GxxTxQK4, GxxUxQK4, GxxVxQK4, GxxWxQK4, GxxXxQK4
|
|
00042100008869 |
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
5 / 1.2 OZ Roll |
Grizzly LC Straight |
GxxQxQK4, GxxRxQK4, GxxSxQK4, GxxTxQK4, GxxUxQK4, GxxVxQK4, GxxWxQK4, GxxXxQK4
|
|
00042100008876 |
This recall is the result of potential adulteration in Grizzly LC Straight Snuff.
Consumers should not consume any remaining product and are advised to return the product to the place of purchase for a full refund.
Fresh Gourmet Tortilla Strips Santa Fe Style
Sugar Foods has issued a voluntary recall on Fresh Gourmet Tortilla Strips Santa Fe Style
People who have an allergy or severe sensitivity to Wheat run the risk of serious allergic reaction if they consume this product.
The Tortilla Strips listed below were distributed through retail stores, and online ecommerce sites.
|
Product size |
Flavor / Lot Code |
Best by |
UPC |
|
|
Tortilla Santa Fe Style |
Santa Fe |
N3-11BHNV3552 |
20JUN2025 |
00787359175046 |
Fresh Gourmet Tortilla Strips Santa Fe Style and must have the best by date listed above printed on the bag.
This voluntary recall is the result of undeclared Wheat in the product.
Consumers who have a Wheat allergy should not eat any remaining product and are advised to return the product to the place of purchase for a full refund.
Consumers with questions may contact sugarfoods.com.
Brookshire’s Frozen Pancakes
Topco has issued a voluntary recall on several batches of Brookshires Frozen Pancakes with the lot numbers listed below due to possible listeria monocytogenes from a supplier.
|
BRK FRZN BTTRMLK PNCKE 16.5 OZ |
38173 |
Lot Code 2C401184 Use By - 1/17/2025 |
00092825096672 |
|
BRK FRZN BTTRMLK PNCKE |
38173 |
Lot Code 2C402294 Use By 2/28/2025 |
92825096672 |
|
BRK FRZN BTTRMLK PNCKE 16.5 OZ |
38173 |
Lot Code 2C405074 Use By 5/7/2025 |
00092825096672 |
|
BRK FRZN BTTRMLK PNCKE 16.5 OZ |
38173 |
Lot Code 2C410233 Use By - 10/22/2024 |
00092825096672 |
|
BRK FRZN BTTRMLK PNCKE |
38173 |
Lot Code 2C412133 Use By - 12/12/2024 |
00092825096672 |
|
BRK FRZN BTTRMLK PNCKES BGD 33 OZ |
38186 |
Lot Code 2C401224 Use By - 1/21/2025 |
00092825096740 |
|
BRK FRZN BTTRMLK PNCKES BGD 33 OZ |
38186 |
Lot Code 2C403264 Use By 3/26/2025 |
00092825096740 |
|
BRK FRZN BTTRMLK PNCKES BGD |
38186 |
Lot Code 2C405314 Use By - 5/31/2025 |
0092825096740 |
|
BRK FRZN BTTRMLK PNCKES BGD 33 OZ |
38186 |
Lot Code 2C406244 Use By - 6/24/2025 |
00092825096740 |
|
BRK FRZN BTTRMLK PNCKES BGD 33 OZ |
38186 |
Lot Code 2C408024 Use By - 8/2/2025 |
00092825096740 |
|
BRK FRZN BTTRMLK PNCKES BGD 33 OZ |
38186 |
Lot Code 2C411153 Use By - 11/14/2024 |
00092825096740 |
|
BRK FRZN BTTRMLK PNCKES BGD |
38186 |
Lot Code 2C412123 Use By - 12/11/2024 |
00092825096740 |
Topco has issued a voluntary recall on several batches of Brookshires Frozen Pancakes with the lot numbers listed below due to possible listeria monocytogenes from a supplier.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Published October 22, 2024
Evol Foods Chicken Enchilada Bake
Conagra Foods has issued a voluntary recall on Evol Foods Chicken Enchilada Bake.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
9OZ |
Evol Chicken Enchilada Bake |
423841 |
June 24 2025 |
891627009022 |
Conagra Foods has issued a recall on Evol Chicken Enchilada Bake
This voluntary recall is the result of possible Listeria in an ingredient.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
9OZ |
Evol Chicken Enchilada Bake |
423841 |
June 24 2025 |
891627009022 |
Consumers should not eat any remaining product and are advised to return the product to the place of purchase for a full refund.
Consumers with questions may contact [email protected]
Published October 21, 2024
Kodiak Vanilla Waffles
Treehouse Foods has issued a voluntary recall on Kodiak Butermilk Vanilla Frozen Waffle due to a potential listeria contamination.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
13.4 OZ |
Kodiak Buttermilk Vanilla Frozen Waffle |
All |
All |
705599012211 |
This voluntary recall is the result of potential listeria contamination
of Kodiak Butermilk Vanilla Frozen Waffles.
Consumers should not consume any remaining product and are advised to return the product to the place of purchase for a full refund.
Consumers with questions may contact [email protected]
Published October 21, 2024
DELI CHICKEN TACO KIT MEALS
Deli Grab and Go Chicken Taco Meal Kit
|
Product Size |
Flavor |
Batch |
UPC |
|
Sold By the Pound |
Deli CHICKEN STREET TACO KIT |
ALL |
13454380826 |
Brookshires has issued a voluntary recall on the Deli Chicken Taco Kit meal due to an ingredient’s potential for listeria.
Consumers should not eat any remaining product and return to Brookshires for a full refund.
Published October 14, 2024
RAO’S FROZEN ALFREDO MEALS
Michael Angelo’s foods has issued a voluntary recall on batches of Rao’s Frozen Chicken Alfredo due to an ingredient’s potential for listeria.
|
Product Size |
Flavor |
Batch |
Best By |
UPC |
|
8.4 OZ |
Rao’s Frozen Chicken Alfredo |
Lot 4215MAG |
2-Aug-26 |
00747479300063 |
|
8.4 OZ |
Rao’s Frozen Chicken Alfredo |
Lot 4242MAG |
29-Aug-26 |
00747479300063 |
|
8.4 OZ |
Rao’s Frozen Chicken Alfredo |
Lot 4250MAG |
6-Sep-26 |
00747479300063 |
|
8.4 OZ |
Rao’s Frozen Chicken Alfredo |
Lot 4263MAG |
19-Sep-26 |
00747479300063 |
Michael Angelo’s foods have issued a voluntary recall on batches of Rao’s Frozen Alfredo meals due to an ingredient’s potential for listeria.
This voluntary recall is the result of a recall due to potential listeria in an ingredient.
Consumers should not eat any remaining product and can contact Michael Angelo’s at 1-877-482- 5426 for a full refund.
Published October 14, 2024
BOSTON MARKET and MICHELINA’S FROZEN MEALS
Bellisio foods have issued a voluntary recall on batches of Boston Market, and Michelinas Frozen meals due to an ingredients potential for listeria.
|
Product Size |
Flavor |
Best By |
UPC |
|
13 OZ |
BM Chicken Alfredo Fettuccini |
Best By: 07/11/25 |
00738912025292 |
|
13 OZ |
BM Chicken Broccoli Cheese |
Best By: 07/25/25 |
|
|
8 OZ |
Michelina’s Pasta Chicken, Pea, & Carrot |
Best By: 08/23/25 |
00717854151092 |
|
8OZ |
Michelina’s Spicy Chicken Alfredo |
Best By 8/30/2025 |
00717854151092 |
|
9oz |
Atkins Chicken Margherita |
Best By: 09/06/25 |
00637480009492 |
Bellisio foods have issued a voluntary recall on batches of Boston Market, and Michelinas Frozen meals due to an ingredient’s potential for listeria.
This voluntary recall is the result of a recall due to potential listeria in an ingredient.
Consumers should not eat any remaining product and are advised to return the product to the place of purchase for a full refund.
Consumers with questions may contact: www.Bellisiofoods.com
Published October 14, 2024
Lactaid Milk 96oz
Hood LLC has issued a voluntary recall on several batches of Lactaid 96 oz milk with the lot numbers listed below due to a potential trace amount of almond.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
96 oz |
Lactaid Whole Milk |
51-4109-P2 |
Nov 22,23,25.26,27,28,2024 Dec2,3,4 2024 |
041383090738 |
|
96 Oz |
Lactaid 2% Milk |
51-4109-P2 |
Nov 23,24,28,29,30 2024 Dec 1 74 2024
|
041383090721 |
WEB Notice
This voluntary recall is the result of a potential of trace amounts of almond in product.
Consumers who have an allergy to almonds should not consume any remaining product and are advised to return the product to the place of purchase for a full refund. If you are not allergic to almond the products are safe for consumption.
Consumers with questions may contact: Hood LLC at 800-242-2423 Monday through Friday from 9 AM – 5 PM Eastern Time.
Published September 20, 2024
P&G Laundry Pods
P&G has issued a voluntary recall in collaboration with CPSC for select lot codes of bags of Tide PODs, Gain Flings, Ace PODs and Ariel PODs distributed in the US and Canada.
Product: Certain lot codes of defective bag packaging of Tide PODs, Gain Flings, Ace PODs and Ariel PODs distributed in the US and Canada between September 2023 – present. No other products are affected except these specific lot codes of bag packaging.
What to Do: Bags from select lot codes sold during this period can have defective closures, which could lead to serious injury if the product inside is able to be accessed unintentionally. Ingestion of a large quantity of any surfactant-containing household cleaning products can cause death among individuals with underlying health issues. If you have an issue closing bag packaging, immediately transfer the product to a container with an effective Child Resistant Closure and store it up, closed and away, since the product inside is unimpacted by this packaging defect. Then, reach out to P&G directly at pggoodeveryday.com/bags or call us at 1-833-347-5764 and our team will be ready to assist you.
Where to find the product code: The lot code can be found on the bottom of the bag. The bar code can be found on the back.
Questions and reimbursement: Consumers who purchased these products can contact our Consumer Care team at 1-833-347-5764 from Monday through Friday, 9 AM ET to 6 PM ET, Saturday, 9 AM ET to 5:30 PM ET, or online at pg.com/bags for a replacement bag package and reimbursement. We apologize for the inconvenience.
Published April 11, 2024
Dietz & Watson has issued a voluntary recall on products containing Coppa, co-packed by Fratelli Beretta USA. due to a potential salmonella contamination. Product information is listed below.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
98833 |
DW PTY PLTR CALABRESE 8 OZ |
No Lot Code |
2/13/2024 - 7/6/2024 |
00031506847103 |
|
98833 |
DW PTY PLTR CALABRESE 8 OZ |
No Lot Code |
2/13/2024 - 7/6/2024 |
50031506847108 |
|
98840 |
DW ANTIPASTO PLATTER 6 OZ |
No Lot Code |
2/13/2024 - 7/6/2024 |
00031506688232 |
|
98840 |
DW ANTIPASTO PLATTER 6OZ |
No Lot Code |
2/13/2024 - 7/6/2024 |
50031506688237 |
|
1467263 |
Italian Hoagie |
Fresh made sandwich from deli. |
2/13/2024 - 7/6/2024 |
00227775000008 |
|
1467260 |
Italian Ciabatta |
Fresh made sandwich from deli, |
2/13/2024 - 7/6/2024 |
00227773000000 |
Published February 14, 2024
Quaker Granola Bars and Cereal
The Quaker Oats Company has expanded the bars and cereals recall. Please see newly added items below, they have the potential to be contaminated with Salmonella.
The Quaker products listed below were distributed nationwide through retail stores, and online ecommerce sites.
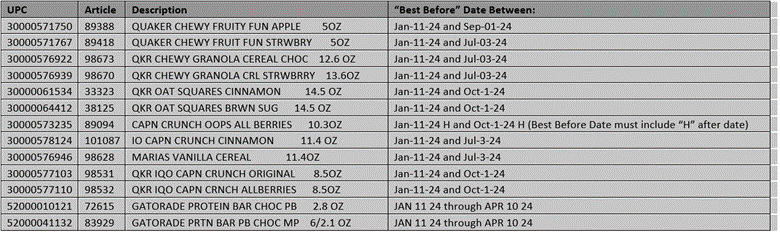
ORIGINAL NOTICE:
The Quaker Oats Company is recalling the specific granola bars and granola cereals listed below because they have the potential to be contaminated with Salmonella. The Quaker products listed below were distributed nationwide through retail stores, and online ecommerce sites.

Published January 15, 2024
Robitussin® Honey CF Max Day/Nighttime Adult product.
This recall has been initiated due to the potential that a product may contain elevated levels of certain strains of yeast. These yeasts, which occur naturally in honey at lower levels, do not generally cause a safety risk to consumers. However, at elevated levels they could impact the product’s taste and odor and cause mild to moderate gastrointestinal symptoms. In addition, the elevated levels of yeast could cause the syrup to carbonate and the plastic bottle to swell.
|
Product size |
Flavor / Lot Code |
Best by |
UPC |
|
|
8Oz |
ROBITUSSIN ADLT COLD FLU MX DAY HONEY |
T08730, T08731, T08732, T08733, T10808 |
31MAY2025 31MAY2025 31MAY2025 31MAY2025 30SEP2025 |
300318771181 |
Robitussin® Honey CF Max Day/Nighttime Adult product.
This recall has been initiated due to the potential that a product may contain elevated levels of certain strains of yeast. These yeasts, which occur naturally in honey at lower levels, do not generally cause a safety risk to consumers. However, at elevated levels they could impact the product’s taste and odor and cause mild to moderate gastrointestinal symptoms. In addition, the elevated levels of yeast could cause the syrup to carbonate and the plastic bottle to swell.
Published January 5, 2024
Quaker Granola Bars and Cereal
The Quaker Oats Company is recalling the specific granola bars and granola cereals listed below because they have the potential to be contaminated with Salmonella. The Quaker products listed below were distributed nationwide through retail stores, and online ecommerce sites.

Published December 15, 2023
Kidfresh Spaghetti Loops
Fairmont Foods has issued a voluntary recall on several batches of Kidfresh Spaghetti Loops with Meat Sauce with the lot numbers listed below due to an undeclared allergen - egg.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
7.25 OZ |
Spaghetti Loops with Meat Sauce |
FF120722, FF011823, FF021623, FF032323, FF042623, FF071923, or FF081023 |
Date ranging from April 2024 to December 2024 on the side of the box. |
00810882010543
|
This voluntary recall is the result of a recall of an undeclared allergen.
Consumers who have an allergy to eggs should not eat any remaining product and are advised to return the product to the place of purchase for a full refund.
Consumers with questions may contact: John Heuer, Executive Vice President, Fairmont Foods, Inc., at 507-238-9001 or [email protected].
Nature’s Path Organic Pumpkin Spice Waffles
Nature’s Path Organic has issued a voluntary recall on several batches of Organic Pumpkin Spice Waffles with the lot numbers listed below due to an undeclared peanut allergen. People who have an allergy or severe sensitivity to peanuts run the risk of serious or life-threatening allergic reaction if they consume these products.
|
Product Size |
Flavor |
Lot Code |
Best By |
UPC |
|
7 .4 oz |
Pumpkin Spice |
2C110242 |
October 24, 2024 |
058449590774 |
This voluntary recall is the result of an undeclared peanut allergen.
Consumers who have an allergy to peanuts should not eat any remaining product and are advised to return the product to the place of purchase for a full refund. If you are not allergic to peanuts the products are safe for consumption.
Consumers with questions may contact Natures Path by calling: 1-866-880-7284
Imagine Chicken Broth
The Hain Celestial Group has issued a voluntary recall on Imagine Chicken Broth, with the lot number listed below due to a lack of documentation on sterility assurance from a co-manufacturer.
|
Product size |
Flavor / Lot Code |
Best by |
UPC |
|
|
32oz |
Imagine Chicken Broth |
Lot Code 98CO7092 |
Best by date 06NOV23 |
084253244244 |
This voluntary recall is the result of a recall that was initiated by a co-manufacturer for a documentation issue. Consumers with questions can contact the Hain company at www.hain.com
Published September 28, 2023
Banquet Chicken Strip Meal
Please be advised Conagra Brands is conducting a product recall for a limited amount of Banquet Chicken Strips Meal. The product may not meet Conagra’s high-quality standards due to the potential presence of a foreign material (plastic). Out of an abundance of caution and to continue to provide a best-in-class consumer experience, Conagra has decided to conduct a product recall for the impacted product.
|
Product size |
Flavor / Lot Code |
UPC |
|
8.9 oz |
Banquet Chicken Meal Lot Codes 5009317120 5009319220 5009319820 |
00-0-31000-00735-5 |
Published September 7, 2023
Knorr Soup Mixes
Unilever United States Inc. is voluntarily recalling select Knorr Sopa Soup Mix products because the products may contain egg, which is not listed as an ingredient on the label. Persons who have an allergy or severe sensitivity to egg run the risk of a serious or life-threatening allergic reaction if they consume these products.
|
The following products are subject to recall: Product |
Lot Code |
Size |
GTIN/UPC |
Case UPC |
|
Knorr Estrellitas con Tomate Tomato Based Star Pasta Soup Mix |
Dates prior to and including July 6, 2024 |
3.5 oz |
048001716193 |
10048001716190 |
|
Knorr Fideos con Tomate Tomato Based Pasta Soup Mix |
Dates prior to and including July 6, 2024 |
3.5 oz |
048001716162 |
10048001716169 |
|
Knorr Letras con Tomate Tomato Based Alphabet Pasta Soup Mix |
Dates prior to and including July 6, 2024 |
3.5 oz |
048001716186 |
10048001716183 |
The recalled products were distributed nationwide. No other Unilever or Knorr products are impacted by this recall. To date, the company has not received any reports of consumer complaints or allergic reactions associated with this product. Anyone concerned about an allergic reaction should contact a healthcare provider.
This limited voluntary recall is being conducted with the knowledge of the U.S. Food and Drug Administration (FDA).
Published August 11, 2023
Belvita Breakfast Sandwich Products
Mondelēz Global LLC announced today a voluntary recall of two varieties of belVita Breakfast Sandwich products, manufactured in the United States and sold nationwide, due to the possibility that the products may contain undeclared peanut resulting from cross-contact on a single manufacturing line. People who have an allergy or severe sensitivity to peanut may risk serious or life-threatening allergic reactions by consuming these products
The Mondelez Global products listed below were distributed nationwide through retail stores, and online ecommerce sites.
|
Product size |
Flavor |
Best by |
UPC |
|
|
belVita Breakfast Sandwich, Dark Chocolate Creme variety (8.8 oz carton) |
Dark Chocolate |
All Best When Used by Dates prior to and including February 25, 2024 (Located on side of carton) |
0 44000 04328 5
|
|
|
belVita Breakfast Sandwich, Dark Chocolate Creme variety (1 lb 5.12 oz carton) |
Dark Chocolate |
All Best When Used by Dates prior to and including February 25, 2024 (Located on side of carton) |
0 44000 05861 6 |
|
|
belVita Breakfast Sandwich, Dark Chocolate Creme variety (14.08 oz carton) |
Dark Chocolate Creme |
All Best When Used by Dates prior to and including February 25, 2024 (Located on side of carton) |
0 44000 06330 6 |
|
|
belVita Breakfast Sandwich, Cinnamon Brown Sugar with Vanilla Creme variety (8.8 oz carton) |
Cinnamon Brown Sugar with Vanilla |
All Best When Used by Dates prior to and including February 25, 2024 (Located on side of carton) |
0 44000 06304 7 |
|
Consumers who have a peanut allergy should not eat any remaining product and are advised to return the product to the place of purchase for a full refund. If you are not allergic to Peanuts the products are safe for consumption.
Consumers with questions may contact Mondelez. at 1-855-535-5948
Published July 5, 2023
Tostitos Avocado Salsa Dip
A Frito Lay. of Plano, TX has issued a voluntary recall of select 15 oz. Tostitos Avocado Salsa with the lot numbers listed below due to an undeclared milk Allergen. People who have an allergy or severe sensitivity to milk run the risk of serious or life-threatening allergic reaction if they consume this product.
This Frito Lay product listed below was distributed nationwide through retail stores, and online ecommerce sites.
|
Product Size |
Lot Code |
UPC |
|
15 oz. |
2 NOV 23 |
15 - 028400055970 |
The product has the correct label on the front but incorrect label on the back.
No illnesses have been reported to date.
This voluntary recall is the result of a recall that was initiated due to the incorrect back label being placed on the back, the front label is correct.
Consumers who have a Milk allergy should not eat any remaining product and are advised to return the product to the place of purchase for a full refund. If you are not allergic to Milk the products are safe for consumption.
Consumers with questions may contact Frito Lay Consumer Relations at 1-800-352-4477 (9 a.m. – 4:30 p.m. CST, Monday-Friday).
Tony Chachere’s Ranch Dressing
A voluntary withdrawal has been announced for Tony Chachere’s Ranch Salad Dressing due to quality concerns of potential product spoilage.
|
Product |
Lot Code |
UPC |
|
Tony Chachere's Ranch Salad Dressing 12 OZ |
LOT# SEC230424 |
71998401018 |
Consumers should dispose of any unused product or return to place of purchase for refund.
Published June 14, 2023Pearl Milling Company Self-Rising Cornmeal Mix
The Quaker Oats Company is issuing a voluntary recall today for specific Best By dates of Pearl Milling Company Self-Rising White Corn Meal Mix (both 80 oz (5 lb) and 30 oz (2 lb) bags) and Pearl Milling Company Self-Rising Yellow Corn Meal Mix (80 oz (5 lb) bags) because they may contain trace amounts of dairy ingredients.
|
Product Description (bag) |
Size |
UPC |
Best By |
|
Pearl Milling Company Self-Rising White Corn Meal Mix |
80 oz (5 lb) |
|
JUL 19 23 |
|
JUL 21 23 |
|||
|
JUL 22 23 |
|||
|
JUL 30 23 |
|||
|
JUL 31 23 |
|||
|
SEP 15 23 |
|||
|
SEP 16 23 |
|||
|
OCT 14 23 |
|||
|
OCT 15 23 |
|||
|
OCT 16 23 |
|||
|
OCT 17 23 |
|||
|
NOV 04 23 |
|||
|
DEC 01 23 |
|||
|
DEC 02 23 |
|||
|
DEC 23 23 |
|||
|
Pearl Milling Company Self-Rising Yellow Corn Meal Mix |
80 oz (5 lb) |
3000057380 |
JUN 20 23 |
|
JUN 21 23 |
|||
|
JUN 22 23 |
|||
|
JUL 22 23 |
|||
|
SEP 04 23 |
|||
|
DEC 05 23 |
|||
|
Pearl Milling Company Self-Rising White Corn Meal Mix |
30 oz (2 lb) |
3000057387 |
JUN 18 23 |
|
JUN 19 23 |
|||
|
JUN 20 23 |
|||
|
AUG 07 23 |
|||
|
AUG 08 23 |
|||
|
OCT 21 23 |
Published June 9, 2023
|
Product |
Pouch Size |
Best By Date(s) |
UPC |
|
Catalina Crunch Honey Graham Cereal |
9oz |
7/25/2023 |
860479001553 |
|
Catalina Crunch Maple Waffle Cereal |
9oz |
7/25/2023 |
860479001539 |
|
9oz |
8/15/2023 |
860479001539 |
|
|
Catalina Crunch Fruity Cereal |
1.27oz |
8/1/2023 |
850017468214 |
|
8oz |
8/1/2023 |
850017468085 |
|
|
Catalina Crunch Chocolate Peanut Butter Cereal |
9oz |
8/1/2023 |
850017468184 |
|
9oz |
8/8/2023 |
850017468184 |
|
|
Catalina Crunch Cheddar Crunch Mix |
6oz |
2/15/2023 |
850017468160 |
|
6oz |
2/22/2023 |
850017468160 |
This recall is being communicated out of an abundance of caution due to potential metal contamination limited to these products with these specific Best By Dates. No injuries or illnesses have been reported to date.
Any product not listed in the above table is not included and not impacted by this recall.
Consumers should not eat product and are advised to destroy or return the product to the place of purchase for a full refund.
Published 2/17/2023
Drizzilicious Mini Rice Cake Bites
Snack Innovations Inc. of Piscataway, NJ has issued a voluntary recall on several batches of Drizzilicious branded mini rice cake bites, and drizzled popcorn products with the lot numbers listed below due to an undeclared peanut residue. People who have an allergy or severe sensitivity to peanuts run the risk of serious or life-threatening allergic reaction if they consume these products.
The Drizzilicious products listed below were distributed nationwide through retail stores, and online ecommerce sites.
|
Product size |
Flavor / Lot Code |
Best by |
UPC |
|
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Birthday Cake |
N3-11BHNV3552 |
21SEP2023 |
4oz - 857900005402 |
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Birthday Cake |
N3-11BHNV3462 |
12SEP2023 |
|
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Birthday Cake |
N3-21BHNV0093 |
09OCT2023 |
|
|
Drizzilicious drizzled Popcorn 3.6oz Bags |
Birthday Cake Popcorn |
N3-11BHNV3322 |
28JUL2023 |
857900005525 |
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Cinnamon Swirl |
N3-11BHNV3562 |
22SEP2023 |
4oz - 857900005167 |
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Cinnamon Swirl |
N4-11BHNV0163 |
16OCT2023 |
|
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Cinnamon Swirl |
N3-11BHNV0053 |
05OCT2023 |
|
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Cookies & Cream |
N4-11BHNV0183 |
18OCT2023 |
4oz - 857900005167 .74oz - 857900005181 |
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Cookies & Cream |
N3-11BHNV0093 |
09OCT2023 |
|
|
Drizzilicious mini rice cake bites 4oz and/or .74oz Bags |
Salted Caramel |
N3-11BHNV0083 |
08OCT2023 |
4oz - 857900005167 |
|
Drizzilicious mini rice cakes 32-ct Variety box |
Variety (3-Flavors) |
N4-11BHNV3562 |
9/22/2023 |
853762002993 |
|
Drizzilicious mini rice cakes 32-ct Variety box |
Variety (3-Flavors) |
N4-21BHNV3532 |
9/19/2023 |
|
The products are packaged in Drizzilicious branded pillow bags and/or stand-up zip pouches and must have one of the lot codes listed above printed on the back of the bag.
No illnesses have been reported to date.
This voluntary recall is the result of a recall that was initiated by an ingredient supplier. The supplier made notification that they discovered undeclared peanut residue in one of the sub-ingredients used in the process of making these products.
Consumers who have a peanut allergy should not eat any remaining product and are advised to return the product to the place of purchase for a full refund. If you are not allergic to Peanuts the products are safe for consumption.
Consumers with questions may contact Snack Innovations Inc. at 1-888-445-5122 or visit their website at https://www.drizzilicious.com/recall/food-safetyExternal Link Disclaimer
Published 2/06/2023
Oysters from TX 1 of Galveston Bay
The Texas Department of State Health Services has ordered a recall for oysters from TX 1 of Galveston Bay harvested from November 17, 2022 through December 7, 2022 due to a potential health hazard. Human consumption of affected product may cause vomiting, diarrhea, nausea and cramping.
Affected product may include, but is not limited to, the following items:
| Description | UPC |
| Misho’s Gulf Oysters 8 OZ | 79312500009 |
| Misho’s Bulk Oysters | N/A |
The Texas Department of State Health Services has been notified that illnesses have been linked to the consumption of oysters harvested from Galveston Bay (TX 1) during the period of November 17, through December 2, 2022.
Published 12/13/2022
Pine-Sol Multi-Surface Cleaners
Pine-Sol has issued a recall on specific scented Pine-Sol cleaners shown below. Consumers should immediately stop using scented Pine-Sol cleaners subject to the recall and visit pinesolrecall.com to request a full refund.
This voluntary recall is being conducted because some products may contain bacteria, including Pseudomonas aeruginosa, an environmental organism found widely in soil and water. People with weakened immune systems or external medical devices who are exposed to Pseudomonas aeruginosa face a risk of serious infection that may require medical treatment. The bacteria can enter the body if inhaled, through the eyes, or through a break in the skin. People with healthy immune systems are usually not affected by the bacteria.
Pine-Sol is conducting this voluntary recall to remove the products from the market out of an abundance of caution to safeguard your health and wellbeing.
Importantly, Original Pine-Sol® Pine scent is not included in this recall and is safe to use.
Consumers who have products matching the following description in their possession should stop using it immediately and visit pinesolrecall.com to request a full refund.
Product safety is a top priority and we’re committed to safeguarding consumers’ health and wellbeing.
| Description | UPC |
| Pine-Sol® Multi-Surface Cleaner Lavender Clean® 28oz | 4129440116 |
| Pine-Sol® Multi-Surface Cleaner Lavender Clean® 48oz | 4129440272 |
| Pine-Sol® Multi-Surface Cleaner Lavender Clean® 60oz | 4129440112 |
| Pine-Sol® Multi-Surface Cleaner Lemon Fresh 28oz | 4129440187 |
| Pine-Sol® Multi-Surface Cleaner Lemon Fresh 48oz | 4129440199 |
| Pine-Sol® Multi-Surface Cleaner Lemon Fresh 60oz | 4129440239 |
| Pine-Sol® Multi-Surface Cleaner Lemon Fresh 175oz | 4129440306 |
| Pine-Sol® Multi-Surface Cleaner Lemon Fresh 100oz | 4129497291 |
| Pine-Sol® Multi-Surface Cleaner Lemon Fresh 2x 100oz | 4129497376 |
| Pine-Sol® Multi-Surface Cleaner Sparkling Wave® 48oz | 4129441904 |
| Clorox Professional Pine-Sol® Lemon Fresh Cleaner 144oz | 4460030891 |
| CloroxPro® Pine-Sol® Lavender Clean® All Purpose Cleaner 144 oz | 4129497301 |
| CloroxPro® Pine-Sol® Lemon Fresh All Purpose Cleaner 144 oz | 4129435419 |
| CloroxPro® Pine-Sol® Orange Energy® All Purpose Cleaner 144 oz | 4129441772 |
| CloroxPro® Pine-Sol® Sparkling Wave® All Purpose Cleaner 144 oz | 4129497434 |
This recall pertains to select scented Pine-Sol products with the UPC codes above with the date code of A422249 and below
Published 10/26/2022
Lactaid Cottage Cheese
HP Hood LLC is voluntarily recalling a select number of containers cottage cheese due to the potential presence of plastic pieces, which they have determined are unlikely to cause serious injuries or illnesses.
This voluntary recall impacts the item listed below carried in Brookshire Grocery Company stores:
| Description | UPC |
| BRIE 3 KG WHEEL | 820581678692 |
BEST BY DATES: 10/24/22, 10/31/22, 11/7/22, 11/14/22, 11/21/22, 11/28/22
This recall does not apply to any other products carried by Brookshire Grocery Company.
Consumer inquiries can be directed to the HP Hood Consumer Affairs hotline at 1-800-242-2423.
Published 10/26/2022
Old Europe Cheese
Old Europe Cheese Inc. of Michigan has issued a Voluntary Recall of the items listed below for potential contamination with Listeria monocytogenes.
| Description | UPC |
| BRIE 3 KG WHEEL | 820581678692 |
| BRIE LA BONNE VIE 8 OZ | 820581678531 |
| BRIE TRIPLE CREME | 820581678746 |
| CAMEMBERT LA BONNE VIE 8 OZ | 820581678616 |
If consumers have products matching the above description in their possession, they should dispose of it immediately, or return for refund. Product should not be ingested.
Published 10/03/2022
Alexia Organic Seasoned Hashbrowns
Lamb Weston has issued a recall out of an abundance of care for the product listed below. They have not received any direct consumer reports of confirmed illnesses related to this product.
| Description | UPC |
| Alexia Organic Seasoned Hashed Browns | 834183003035 |
The recall was initiated due to the potential presence of Listeria monocytogenes, which was discovered during sampling of the product. Listeria monocytogenes is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, listeria infection can cause miscarriages and stillbirths among pregnant women.
Although no illnesses have been identified in connection with the products, consumers should not eat any of the recalled items, as they could be contaminated with Listeria monocytogenes. While these products are not ready-to-eat items and have cooking instructions which, if followed, will reduce consumer risk, there remains some risk that the mishandling of this product prior to or without adequate cooking may cause illness.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Published 8/25/2022
King’s Hawaiian Pretzel Buns
King’s Hawaiian is voluntarily recalling its Pretzel Slider Buns, Pretzel Hamburger Buns and Pretzel Bites products out of an abundance of caution following a recall of an ingredient used in the pretzel products from one of its suppliers, Lyons Magnus. Lyons Magnus is recalling this ingredient due to the potential for it to cause microbial contamination including from the organisms Cronobacter sakazakii and Clostridium botulinum. While no illnesses associated with King’s Hawaiian pretzel bread have been reported, and no pathogens have been found in any King’s Hawaiian products to date, the recall is being conducted to ensure consumer safety.
This recall does not impact any other King’s Hawaiian products, as no other products use this ingredient from Lyons Magnus. King’s Hawaiian will resume producing all pretzel products once the company has ensured all current product has been disposed of and has confirmed the safety of all ingredients.
Consumers in possession of any King’s Hawaiian Pretzel Slider Buns, King’s Hawaiian Pretzel Hamburger Buns or King’s Hawaiian Pretzel Bites should dispose of the product. Consumers can contact King’s Hawaiian at 877-695-4227, Monday through Friday from 8:30 a.m. to 5:00 p.m. PT, if they have any questions, or to request replacement product.
Published 8/16/2022
Premier Protien Shakes
Lyons Magnus LLC announced that it is voluntarily recalling nutritional and beverage products due to the potential for microbial contamination, including from the organism Cronobacter sakazakii. The list of recalled products does not include products intended for infants (i.e., under the age of one). While infection related to Cronobacter sakazakii is rare, the common symptoms of illness could include fever, vomiting and urinary tract infection. However, vulnerable and immunocompromised populations may be more susceptible to infection. To date, no illnesses or complaints related to these products have been reported. The recalled products should not be consumed.
Preliminary root cause analysis shows that the products did not meet commercial sterility specifications.
The following items/lots were shipped to Brookshire Grocery Company stores. For a complete listing of all products and lot codes affected by this recall please visit www.lyonsmagnus.com.
| Description | UPC |
| Premier Protein® Vanilla High Protein Shakes 11 oz carton | 00643843714514 |
| Premier Protein® Café Latte High Protein Shakes 11 oz carton | 00643843716679 |
Anyone who has a recalled product in their possession should dispose of it immediately or return it to the place of purchase for a refund. Consumers in all time zones with questions may contact the Lyons Recall Support Center 24/7 at 1-800-627-0557, or visit its website at www.lyonsmagnus.com.
This recall is being conducted in cooperation with the U.S. Food and Drug Administration.
Published 7/29/2022
TopCare Magnesium Citrate Saline Laxative Oral Solution Cherry Flavor
A Product Recall has been issued by Topco Associates for TopCare Magnesium Citrate Saline Laxative Oral Solution Cherry Flavor, 10 FL OZ (296 mL) due to the presence of the pathogen Gluconacetobacter liquefaciens. This is a safety issue. Immunocompromised patients, who consume this product, may be at increased risk for infections caused by Gluconacetobacter liquefaciens that could lead to serious, life-threatening adverse health consequences.
All lots of product are affected by this recall.
| Description | UPC |
| TopCare Magnesium Citrate Saline Laxative Oral Solution Cherry Flavor | 00036800455306 |
If consumers have products matching the above description in their possession, they should dispose of it immediately, or return for refund. Product should not be ingested.
Published 7/22/2022
TopCare Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor
A Product Recall has been issued by Topco Associates for TopCare Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor, 10 FL OZ (296 mL) due to the presence of the pathogen Gluconacetobacter liquefaciens. This is a safety issue. Immunocompromised patients, who consume this product, may be at increased risk for infections caused by Gluconacetobacter liquefaciens that could lead to serious, life-threatening adverse health consequences.
All lots of product are affected by this recall.
| Description | UPC |
| TopCare Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor | 00036800455290 |
If consumers have products matching the above description in their possession, they should dispose of it immediately, or return for refund. Product should not be ingested.
Published 7/15/2022
BROOKSHIRE GROCERY COMPANY RECALLS YELLOW FLESH PEACHES BECAUSE OF POSSIBLE HEALTH RISK
Brookshire Grocery Company of Tyler, Texas has issued a voluntary recall of bulk Yellow Flesh Peaches available in stores between 4/15/22 and 5/17/22, because they have the potential to be contaminated with Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.
Yellow Flesh Peaches subject to this voluntary recall were sold at Brookshire’s, Super 1 Foods, Spring Market, and FRESH by Brookshire’s retail stores in Texas, Louisiana, and Arkansas.
The Yellow Flesh Peaches are a product of Chile and may have a PLU sticker with the words “CHILE” and “TREE RIPE YELLOW PEACH” and the numeral 4044. Potentially affected product was received from a distributor and shipped to store locations between 4/15/22 and 4/24/22. Due to the fresh nature of the product, no fresh fruit is expected to be in any household, but consumers who may have frozen or otherwise preserved this item may have it in their possession.
No illnesses have been reported to Brookshire Grocery Company to date.
The recall is a result of random sampling conducted at Brookshire’s distribution center by the Texas Department of State Health Services after potentially affected product was shipped to stores and which revealed a positive test for Listeria monocytogenes. Brookshire Grocery Company immediately disposed of the affected product at the distribution center, issued a recall notice to its stores, and implemented sanitation procedures at all retail and affected locations.
Any consumer who may have purchased bulk Yellow Flesh Peaches from a Brookshire Grocery Company retail store between 4/15/22 and 5/17/22 and still has them in their possession should dispose of the product immediately. Consumers with questions may contact Brookshire Grocery Company at 1-888-937-3776.
Published 5/25/2022
Jif Peanut Butter
The J. M. Smucker Co. is recalling select Jif® peanut butter products sold in the U.S. due to potential Salmonella contamination. Salmonella is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis.
The recalled peanut butter was distributed nationwide in retail stores and other outlets.
Recalled products include products with lot codes where the first four digits are between 1274 and 2140, and if the next three numbers after that are '425', this product has been recalled and you should not consume this product.
The lot code can be located on the back of the jar, under the Best If Used By Date (the lot code may be next to the Best If Used By Date for cups or squeeze pouches).
Description and UPC
- JIF 16 OUNCE CREAMY PEANUT BUTTER 5150025516
- JIF 16 OUNCE CRUNCHY PEANUT BUTTER 5150025537
- JIF 96 OUNCE CREAMY PEANUT BUTTER TWIN PACK 5150024705
- JIF 96 OUNCE CRUNCHY PEANUT BUTTER TWIN PACK 5150024706
- JIF 40 OUNCE NATURAL CRUNCHY PEANUT BUTTER 5150007565
- JIF 12 OUNCE CRUNCHY PEANUT BUTTER INTERNATIONAL 5150008026
- JIF 3/4 OUNCE PEANUT BUTTER PLASTIC CASE 5150008051
- JIF .64 OUNCE NATURAL PEANUT BUTTER PLASTIC CASE 5150008058
- JIF 96 COUNT NATURAL PEANUT BUTTER TO GO CASE 5150021889
- JIF 36 COUNT CREAMY JIF PEANUT TO GO CASE 5150024114
- JIF 8 COUNT CRUNCHY PEANUT BUTTER TO GO 5150024130
- JIF 8 COUNT CREAMY PBTR TO GO 5150024136
- JIF 4.5 OUNCE CREAMY PEANUT BUTTER TO GO 5150024137
- JIF 54 OUNCE CREAMY PEANUT BUTTER TO GO 36 PACK 5150024143
- JIF 28 OUNCE CRUNCHY PEANUT BUTTER 5150024163
- JIF 96 COUNT CREAMY PEANUT BUTTER TO GO 5150024170
- JIF 54 OUNCE NATURAL CREAMY PEANUT BUTTER TO GO 36 PACK 5150024174
- JIF 28 OUNCE CREAMY PEANUT BUTTER 5150024177
- JIF 40 OUNCE NATURAL HONEY 5150024182
- JIF 12 OUNCE CREAMY PEANUT BUTTER 5150024191
- JIF 12 OUNCE NATURAL CREAMY PEANUT BUTTER TO GO 5150024307
- JIF 40 OUNCE NATURAL CREAMY PEANUT BUTTER 5150024321
- JIF 28 OUNCE NATURAL CREAMY PEANUT BUTTER 5150024322
- JIF 4 POUND CAN CREAMY PEANUT BUTTER 5150024331
- JIF 96 OUNCE NATURAL CREAMY TWINPACK 5150024404
- JIF 15.5 OUNCE NO ADDED SUGAR PEANUT BUTTER 5150024540
- JIF 13 OUNCE SQUEEZABLE POUCH 5150024545
- JIF 33.5 OUNCE NO ADDED SUGAR PEANUT BUTTER 5150024548
- JIF 13 OUNCE NATURAL SQUEEZE POUCH 5150024572
- JIF 80 OUNCE CREAMY PEANUT BUTTER TWIN PACK 5150024769
- JIF 80 OUNCE CRUNCHY PEANUT BUTTER TWIN PACK 5150024776
- JIF 40 OUNCE REDUCED FAT CREAMY PEANUT BUTTER 5150025499
- JIF 16 OZ REDUCED FAT CREAMY PEANUT BUTTER 5150025518
- JIF 16 OUNCE CREAMY OMEGA 3 PEANUT BUTTER 5150025530
- JIF 80 OUNCE NATURAL CREAMY PEANUT BUTTER TWIN PACK 5150025542
- JIF 16 OUNCE NATURAL CREAMY PEANUT BUTTER 5150025565
- JIF 16 OUNCE NATURAL CRUNCHY PEANUT BUTTER 5150025574
- JIF 16 OUNCE NATURAL CREAMY PEANUT BUTTER HONEY 5150025578
- JIF 40 OUNCE CREAMY PEANUT BUTTER 5150072001
- JIF 40 OUNCE CRUNCHY PEANUT BUTTER 5150072002
- JIF 46.5 OUNCE NO ADDED SUGAR PEANUT BUTTER 5150041418
- JIF 1.1 OUNCE PORTION CONTROL PEANUT BUTTER 120 COUNT 5150092100
- JIF 48 OUNCE CREAMY PEANUT BUTTER 5150024094
- JIF 48 OUNCE CRUNCHY PEANUT BUTTER 5150024095
- JIF 1.5 oz CREAMY PEANUT BUTTER TO GO 5150024141
- JIF 48 OUNCE NATURAL CREAMY 5150024402
- JIF 40 OUNCE CREAMY PEANUT BUTTER 5150024090
- JIF 40 OUNCE CRUNCHY PEANUT BUTTER 5150024091
- JIF 40 OUNCE NATURAL CREAMY PEANUT BUTTER 5150025524
If consumers have products matching the above description in their possession, they should dispose of it immediately.
Consumers who have questions or would like to report adverse reactions should visit www.jif.com/contact-us or call 800-828-9980 Monday through Friday, 8 AM to 5 PM ET.
This recall is being conducted in cooperation with the U.S. Food and Drug Administration.
Published 5/24/2022
Brookshire's Frozen Pizzeria Crust Chicken Bacon Ranch Pizza
Topco Associates has issued a recall for Brookshire's Frozen Pizzeria Crust Chicken Bacon Ranch Pizza due to the potential presence of a foreign material, specifically metal, in the bacon topping.
| Product | UPC | Best By Date |
| Brookshire's Frozen Pizzeria Crust Chicken Bacon Ranch Pizza | 092825106753 | 12/5/2022 |
Affected product should not be consumed, but should be disposed of/destroyed or returned for refund.
Published 5/24/2022
LIFESAVERS Gummies
Mars Wrigley Confectionery US, LLC announced a voluntary recall of specific varieties SKITTLES® Gummies, STARBURST® Gummies, and LIFE SAVERS® Gummies due to the potential presence of a very thin metal strand embedded in the gummies or loose in the bag. They received reports from consumers alerting them to this matter and are not aware of any illnesses to date.
The products subject to this recall include specific varieties of SKITTLES® Gummies, STARBURST® Gummies, and LIFE SAVERS® Gummies are described in the table below. On the back of the package is a 10-digit manufacturing code; the first three digits in this code will indicate implicated product as described in the table below (please note, Brookshire Grocery Company carried only the LIFE SAVERS® items in Brookshire’s, Spring Market, Super 1 Foods, and FRESH by Brookshire’s stores):
| Product | UPC | Code (first three digits) |
|
STARBURST® Gummies Original Share Size 3.5oz |
10022000253092 | 136, 139, 140 |
|
STARBURST® Gummies Original Peg Pack 5.8oz |
10022000253818 00022000284648 |
136, 139, 140 |
|
STARBURST® Gummies Sours Share Size 3.5oz |
10022000253122 | 134,135, 137-142 |
|
STARBURST® Gummies Sours Peg Pack 5.8oz |
10022000253801 00022000284617 10022000259384 |
134,135, 137-142 |
|
STARBURST® Gummies Sour Berries Peg Pack 5.8oz |
00022000284624 | 135, 138, 139 |
|
LIFE SAVERS® Gummies Five Flavor Peg Pack 7.0oz, 3.22oz |
10022000285277 10019000083422 10022000285291 |
136, 139 |
|
LIFE SAVERS® Wild Berries Gummies Peg Pack 7.0 oz |
10019000083446 10022000244502 |
136 – 138, 140, 147, 149 - 152 |
|
LIFE SAVERS® Sour Gummies Peg Pack 7.0 oz, 180g |
10022000242058 10022000244533 00019000170491 |
132-134, 139-140, 144-147, 149, 151, 152, 201 |
|
SKITTLES® Gummies Original Peg Pack 5.8 oz, 2.93oz |
10022000285956 00022000286727 10022000287363 |
139 - 218 |
|
SKITTLES® Gummies Original Stand Up Pouch 12oz |
10022000287325 00022000287434 |
139 - 218 |
| SKITTLES® Wild Berry Gummies Peg Pack 5.8 oz, 2.93oz |
10022000285970 00022000286734 10022000287387 |
138 - 218 |
|
SKITTLES® Gummies Wild Berry Stand Up Pouch 12oz |
10022000287349 00022000287441 |
138 - 218 |
| SKITTLES® Sour Gummies Peg Pack 5.8 oz |
10022000289749 00022000291073 00022000289735 |
204 - 218 |
Affected product should be disposed/destroyed and not consumed or returned to place of purchase for refund.
Published 5/17/2022
Abbott Powdered Formula Recall
Abbott is initiating a proactive, voluntary recall of powder formulas, including Similac®, Alimentum® and EleCare® manufactured in Sturgis, Mich., one of the company’s manufacturing facilities. The recall does not include any metabolic deficiency nutrition formulas.
Abbott is voluntarily recalling these products after four consumer complaints related to Cronobacter sakazakii or Salmonella Newport in infants who had consumed powder infant formula manufactured in this facility.
Importantly, no distributed product has tested positive for the presence of either of these bacteria, and Abbott conducts extensive quality checks on each completed batch of infant formula, including microbiological analysis prior to release. All finished products are tested for Cronobacter sakazakii, Salmonella Newport and other pathogens and they must test negative before any product is released. Additionally, retained samples related to the three complaints for Cronobacter sakazakii tested negative for Cronobacter sakazakii. And the retained sample related to the complaint for Salmonella Newport tested negative for Salmonella Newport.
While Abbott's testing of finished product detected no pathogens, they are taking action by recalling the powder formula manufactured in this facility with an expiration of April 1, 2022, or later. No Abbott liquid formulas, powder formulas, or nutrition products from other facilities are impacted by the recall.
To find out if the product you have is included in this recall, visit similacrecall.com and type in the code on the bottom of the package, or call 1-800-986-8540 and follow the instructions provided.
If your product is affected by the recall, do not use it and go to similacrecall.com for a refund or replacement. You can also call Similac customer service at 1-800-986-8540.
Published 2/18/2021
Abbott Powdered Formula Recall
Published 2/18/22
Panera Chicken Tortilla Soup
Blount Fine Foods, a McKinney, Texas establishment, is recalling approximately 6,384 pounds of chicken tortilla soup products that may be contaminated with extraneous material, specifically pieces of gray nitrile glove.
The fully cooked, ready to eat, chicken tortilla soup was produced on July 1, 2021. The following products are subject to recall View Label
- 16-oz. – Plastic containers of “Panera BREAD at HOME Chicken Tortilla Soup” with lot code 070121-1V and “Use By 09/09/2021” on the label.
The products subject to recall bear establishment number “P-13130” inside the USDA mark of inspection. These items were shipped to retail locations in Arizona, Florida, Georgia, and Texas.
The problem was discovered after the company notified FSIS that they received several consumer complaints reporting pieces of gray nitrile glove in the product.
There have been no confirmed reports of adverse reactions due to consumption of these products. Anyone concerned about an injury or illness should contact a healthcare provider.
Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase.
Consumers with questions about the recall can contact Blount Fine Foods Customer Care Team at (866) 674-4519 Monday – Friday from 9 AM to 9 PM Eastern Standard Time.
Published 8/10/2021
Tyson Foods Voluntarily Recalls Frozen, Fully Cooked Chicken
Tyson Foods, Inc, is voluntarily recalling approximately 8.5 million pounds of frozen, fully cooked chicken. Tyson has been working closely with the U.S. Department of Agriculture on this recall, and while there is no conclusive evidence that the products were contaminated at the time of shipment, the voluntary recall is being initiated out of an abundance of caution.
The affected products were produced at one plant located in Dexter, Missouri, between December 26 of 2020 and April 13 of 2021 and distributed to foodservice and retail customers nationwide and Puerto Rico. They are being recalled as a precaution due to possible exposure to Listeria monocytogenes, a harmful bacteria.
“We’re committed to providing safe, healthy food that people rely on every day,” said Scott Brooks, senior vice president, food safety and quality assurance, Tyson Foods. “We are taking this precautionary step out of an abundance of caution and in keeping with our commitment to safety.”
Products Included in this Recall
The recall includes Tyson® branded frozen, fully cooked products as well as private label products made for customers. These products were sold to foodservice and retail customers and distributed nationwide. Each package of the affected retail products has the establishment code P-7089.
A list of product labels for the impacted retail products are available for download and comprehensive list of all retail and foodservice products can be found here.
Photos of the impacted retail products can be downloaded as a PDF or ZIP file.
Only those products listed are being recalled. No other Tyson products are impacted by the recall, including but not limited to any Tyson® brand fresh chicken; frozen, raw chicken products or chicken nuggets.
A list of retail stores that received the product will eventually be posted on USDA’s website. Use the following link to locate the “retail distribution list.”
https://www.fsis.usda.gov/recalls
Consumers with questions should call or text 1-855-382-3101. Customer service representatives will be available beginning Sunday through Friday 8am – 5pm CDT.
Published 7/7/2021
Evolve Plant-Based Protein Shake
CytoSport Inc. has issued a voluntarily recall for Evolve 11 oz. Chocolate Protein Shake in Tetra Pak cartons and Evolve 11 oz. Vanilla Bean in Tetra Pak cartons (please see product list below) due to the potential presence of an allergen (a small amount of soy protein).
This recall is being conducted with the knowledge of the Food and Drug Administration.
No confirmed reports have been received of any consumer illness nor injuries to date.
|
Product |
Package |
"Best Before" Date |
Production Code |
|
Evolve Double Chocolate Protein Shake |
11 oz - single serve Tetra Pak cartons |
DEC 31 21 |
DEC 31 21 ###### |
|
XXXXXU12310 |
|||
|
Evolve Double Chocolate Protein Shake |
11 oz - single serve Tetra Pak cartons |
JAN 01 22 |
JAN 01 22 ###### |
|
XXXXXU01011 |
|||
|
Evolve Vanilla Bean Protein Shake |
11 oz - single serve Tetra Pak cartons |
DEC 30 21 |
DEC 30 21 ###### |
|
XXXXXU12300 |
|||
|
Evolve Vanilla Bean Protein Shake |
11 oz - single serve Tetra Pak cartons |
JAN 25 22 |
JAN 25 22 ###### |
|
XXXXXU01251 |
Consumers are urged not to use recalled product.
Recalled product can be returned to the place of purchase for a full refund or consumers may call CytoSport Customer Relations at 1-888-298-6629.
Published 4/30/2021
Excedrin Recall
GSK Consumer Healthcare has asked retailers to stop selling specific lots of Excedrin due to the possibility of holes in the bottles that have been supplied by the bottle manufacturer.
This recall involves 50, 80, 100, 125, 200, 250 and 300-count bottles of Excedrin Migraine Caplets, Excedrin Migraine Caplets/Geltabs, Excedrin Extra Strength Caplets, Excedrin PM Headache Caplets, and Excedrin Tension Headache Caplets. The bottles are plastic with a child-resistant closure.
Product NOT impacted: 24-count bottles of Excedrin Extra Strength caplets and Excedrin Migraine caplets and geltabs.
Consumers should immediately store the recalled Excedrin bottles out of sight and reach of children and inspect the bottom of the bottle to determine if there is a hole. If there is a hole in the bottle, contact GSK Consumer Relations for information on how to receive a prepaid shipping label for return to receive a full refund. Bottles without a hole can be retained and used as directed.
If you have additional questions, or require further assistance, please contact the GSK Consumer Relations Team on: 1-800-468-7746, Monday – Friday, 8;00am – 6:00pm EST
Published 12/23/2020
That’s Smart American Flavor Sandwich Slices Imitation Process Cheese Food
Topco Associates has issues a market withdrawal of the product listed below due to foreign material contamination, specifically individual units may contain soft plastic pieces. This is not a choking hazard nor is it a food safety issue.
|
Item |
UPC |
Sell/Use By Date |
|
That’s Smart American Flavor Sandwich Slices Imitation Process Cheese Food |
193476000442 |
8/25/2021 |
Consumers should dispose of any unused product or return it to the place of purchase for a refund.
Published 12/11/2020
Full Circle Organic Microwave Popcorn
The specific date/lot of the items listed below is being recalled due to potential foreign material (glass) contamination.
|
Item UPC / Num |
Description |
Lot Code(s) |
Sell/Use By Date(s) |
|
36800406094 |
Full Circle Organic Microwave Butter Popcorn 3.3 OZ |
JUL1921N2 |
7/19/2021 |
|
JUL2021N2 |
7/20/2021 |
||
|
36800406117 |
Full Circle Organic Microwave No Butter Popcorn 2.9 OZ |
JUL3121N2 |
7/31/2021 |
Consumers should not use this product. They should dispose of it or return it for a refund.
Published 9/09/2020
Paws Happy Life Butchers Choice Dog Food
The specific date/lot of the item listed below is being withdrawn from sale due to the potential contamination with aflatoxin.
|
Item UPC / Num |
Description |
Lot Code |
Sell/Use By Date |
|
036800357631 |
Paws Happy Life Butchers Choice Dog Food |
R2_040420 |
4/4/2021 |
Consumers should not use this product. They should dispose of it or return it for a refund.
Published 9/02/2020
Peaches
Out of an abundance of caution, BGC is removing from sale all yellow, white, and organic peaches due to a recently announced recall. Peaches purchased through Saturday August 22nd 2020 are potentially affected by this recall.
Customers are urged not to consume any peaches purchased before 8/23/2020 and should either dispose of them or return them for a refund.
Consumers with questions may contact Prima Wawona’s toll-free number at 1-877-722-7554 or visit its website at www.wawonapacking.com for more information.
Published 8/24/2020
José Olé Taquitos
The U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) is issuing a public health alert for frozen ready-to-eat beef and chicken taquitos products containing Food and Drug Administration (FDA) regulated diced green chilies. The chilis have been recalled by the producer, Sun Valley Foods, due to concerns that the products may be contaminated with extraneous materials, specifically hard plastic. The hard plastic may pose a choking hazard or cause damage to teeth or gums.
The Ajinomoto Foods North America, Inc. (AFNA) products that are subject to the public health alert and were received by Brookshire Grocery Company are as follows:
• José Olé Taquitos Beef Carne De Res In Corn Tortillas UPC 073202894517 with a best by date of “08 JUL 2021”.
• José Olé Taquitos Chicken And Cheese Pollo Y Queso In Flour Tortillas UPC 073202894579 with a best by date of “09 JUL 2021,” “14 JUL 2021” or “17 JUL 2021”.
Do not consume affected product. Please dispose of affected product rendering it inedible or return it for a refund.
Published 8/03/2020
Healthy Choice POWER BOWLS Expanded Recall
Conagra Brands is voluntarily recalling 34,173 cases of a variety of Healthy Choice Breakfast Power Bowls and 26,332 cases of Healthy Choice Power Bowl Chicken Feta and Farro due to the potential presence of an extraneous material (small rocks/stones). This recall is an expansion of the April 10, 2020 recall in which small pebbles or rocks were inadvertently left in harvested ingredients used in a limited quantity of Healthy Choice Power Bowls.
|
Product Description |
UPC |
Batch/Lot Code |
Best By Date |
|
HC CKN FETA FARRO PWR BWL 9.5 OZ |
00072655001800 |
5006006620 |
12/1/2020 |
|
HC TKY SAUS EGG SCRMBL PWR BWL 7.2 OZ |
00072655000810 |
5009003020 |
10/26/2020 |
|
HC UNWRPD BUR SCRMBL PWR BWL 7.2 OZ |
00072655000827 |
5009002920 |
10/25/2020 |
Consumers with questions about the recall or seeking a refund should contact Conagra Brands Consumer Care at 1-800-672-8240 or at [email protected].
Published 5/25/2020
Banquet Mega Sandwich Pepperoni Stuffed Pizza
Conagra Brands is voluntarily recalling 2,339 cases of Banquet Mega Sandwich Pepperoni Stuffed Pizza product due to the potential presence of an undeclared soy allergen in the product. The presence of this undeclared allergen occurred due to a limited number of meatball sandwiches containing soy being inadvertently packed into the Banquet Mega Sandwich Pepperoni Stuffed Pizza packaging.
There have been no reports of adverse allergic reactions due to consumption of this product. Consumers with questions about the product should contact Conagra Brands Consumer Care at 1-800-672-8152 from 9AM-5PM CST Monday through Friday.
The following product is subject to recall:
- Banquet Mega Sandwich Pepperoni Stuffed Pizza 10 OZ, UPC 036800286412, with a Batch/Lot code 5659915510 and “Best if Used By” date MAY/29/2020.
Consumers are urged not to consume the recalled product. Product should be disposed of or returned to place of purchase for refund. Consumers with questions about the product should contact Conagra Brands Consumer Care at 1-800-672-8152 from 9AM-5PM CST Monday through Friday.
Published 1/31/2020
Eggland's Best Snacks
Eggland’s Best, LLC has issued a voluntary recall on the items listed below that have been linked to a hard-boiled egg recall by supplier, Almark Foods. Almark Foods has recently expanded a recall initially announced on December 20th, 2019 in connection to a Listeria monocytogenes outbreak.
The following product is subject to recall:
- Eggland’s Best Hard-Cooked Egg, Bacon & Cheddar Snack, UPC 715141216066
- Eggland’s Best Hard-Cooked Egg, Salame & Provolone Snack, UPC 715141216073
Consumers are urged not to consume the recalled product. Product should be disposed of or returned to place of purchase for refund.
Published 12/27/2019
Food Club Hard Cooked Eggs
A Voluntary Recall has been issued by Topco Associates out of an abundance of caution per their supplier for Food Club Hard Cooked Eggs due to a positive environmental swab for Listeria monocytogenes in the production facility.
The following product is subject to recall:
Food Club Hard Cooked Eggs 6 count, UPC 036800286412
Consumers are urged not to consume the recalled product. Product should be disposed of or returned to place of purchase for refund.
Published 12/24/2019
Mann’s Vegetable Products Recall
Mann Packing Co., Inc. has announced the voluntary recall of several vegetable products sold in the United States and Canada. The voluntary recall is a response to a notification by the Food and Drug Administration and the Canadian Food Inspection Agency of a potential contamination with Listeria monocytogenes. To date, public health officials have not reported any illness associated with these products.
Mann Packing is issuing this recall out of an abundance of caution. Mann Packing is working closely with the authorities to investigate the issue.
The recalled products have “Best If Enjoyed By” date of October 11, 2019 to November 16, 2019.
The full list of products and all corresponding product images are available at:
https://mannpackingproductlist11-2019.us
Consumers who believe that they are in possession of any of the products affected by this recall should dispose of the product in an appropriate waste container.
For any inquiries or comments, all consumers should call Mann’s 24 hour customer service line at 1-844-927-0707 or email Mann Packing Co., Inc. at [email protected]
Published 11/4/2019
Market Sandwich® Brand Sandwiches and Wraps
E.A. Sween Company announced the recall of multiple products due to possible contamination of Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.
Tip Top Poultry, Inc., initiated a recall and is an ingredient provider to two of E.A. Sween’s suppliers, The Suter Company, Inc. that provides chicken salad products and Baja Foods LLC that provides burritos, to the company. To date there have been no reported illnesses related to these items.

Customers with questions are asked to call their Customer Service hotline at 1-800-328-8184 and select #6 for information and refund instructions.
Published 10/7/19
Gold Medal Unbleached All Purpose 5 LB Flour
General Mills has announced a voluntary recall involving Gold Medal Unbleached All Purpose 5lb Flour with UPC 000-16000-12690-9 and “Better if Used by” date 06SEPT2020KC due to the potential presence of E. coli. Consumers are urged to dispose of the product or return it for a full refund.
This impacts only Gold Medal Unbleached All Purpose 5lb flour. No other types or sizes of flour are included.
Consumer inquires can be directed to a special hotline at 1 (800) 230-8103.
No illnesses have been reported in conjunction with this recall.
Published 9/16/19
Lay’s Lightly Salted Barbecue Flavored Potato Chips
Frito-Lay has announced a limited voluntary recall of 7 3/4 oz. bags of Lay’s Lightly Salted Barbecue Flavored Potato Chips because they may contain undeclared milk ingredients. People who have an allergy or severe sensitivity to milk run the risk of a serious or life-threatening allergic reaction if they consume the product contained inside the recalled potato chips bags.
Recalled Product Information

No other Lay’s products are impacted. No other size Lay’s Lightly Salted Barbecue sizes are impacted.
Consumers with any recalled products should contact Frito-Lay Consumer Relations at 1-800-352-4477 (9 a.m. – 4:30 p.m. CST, Monday-Friday).
Published 6/17/19
Tyson Chicken Strips
Tyson Foods, Inc. is recalling approximately 11,829,517 million pounds of frozen, ready-to-eat chicken strip products that may be contaminated with extraneous materials, specifically pieces of metal.
The frozen, ready-to-eat chicken strip items were produced on various dates from Oct. 1, 2018 through March 8, 2019 and have “Use By Dates” of Oct. 1, 2019 through March 7, 2020. The chart contains a list of the products subject to recall. View Labels
The products subject to recall bear establishment number “P-7221” on the back of the product package. These items were shipped to retail and Department of Defense locations nationwide, for institutional use nationwide and to the U.S. Virgin Islands.
The problem was discovered when FSIS received two consumer complaints of extraneous material in the chicken strip products. FSIS is now aware of six complaints during this time frame involving similar pieces of metal with three alleging oral injury.
Anyone concerned about an injury or illness should contact a healthcare provider.
Published 5/6/19
Chips Ahoy Chewy Cookie
Mondelēz Global LLC announced today a limited voluntary recall in the United States of certain Chewy Chips Ahoy 13oz cookies. This voluntary recall is being conducted because of the potential for certain product to contain an unexpected solidified ingredient. Some reports of potential adverse health effects have been received. This recall is limited exclusively to the products listed in the table below, available at retail stores nationwide. No other Chips Ahoy or Mondelēz Global LLC product is included in this recall.
| Description | Retail UPC | Best When Used by Date |
| Chips Ahoy Chewy Cookie (13 oz) | 0 44000 03223 4 | 07SEP2019 08SEP2019 14SEP2019 15SEP2019 (Located on left top side of package by lift tab) |
Published 4/15/19
Jennie-O Ground Turkey
Jennie-O Turkey Store Sales, LLC, is recalling approximately 91,388 pounds of raw ground turkey products that may be associated with an illness outbreak of Salmonella Reading, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced. The raw ground turkey products were produced on September 11, 2018. The following products are subject to recall:
- 1-lb. packages of “Jennie-O GROUND TURKEY 93% LEAN | 7% FAT” with “Use by” dates of 10/01/2018 and 10/02/2018.
- 1-lb. packages of “Jennie-O TACO SEASONED GROUND TURKEY” with a “Use by” date of 10/02/2018.
- 1-lb. packages of “Jennie-O GROUND TURKEY 85% LEAN | 15% FAT” with a “Use by” date of 10/02/2018.
- 1-lb. packages of “Jennie-O ITALIAN SEASONED GROUND TURKEY” with a “Use by” date of 10/02/2018.
The products subject to recall bear establishment number “P-190” inside the USDA mark of inspection. These items were shipped to retail locations nationwide. Brookshire Grocery Company received only a small number of cases of “Jennie-O GROUND TURKEY 93% LEAN | 7% FAT” product that may be affected. This specific recall does not affect any other Jennie-O products. Some product may be frozen and still in consumers’ freezers. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase. Consumers with questions regarding the recall can contact Jennie-O Consumer Engagement Team at 1-800-621-3505, 8 a.m. – 4 p.m. Central Time Monday – Friday and 9 a.m. – 5 p.m. Central Time Saturday and Sunday.
Published 11/16/18
Steam'ables Asparagus Spears Recall
The Pictsweet Company has recalled 1,872 cases of Pictsweet Farms 8-ounce Steam'ables Asparagus Spears because they have the potential to be contaminated with Listeria monocytogenes. The recall affects only Pictsweet Farms 8-ounce Steam'ables Asparagus Spears identified by UPC code O 70560 97799 9 with production codes beginning with the following six digits: 2138XD and a "BEST BY AUG 1, 2020." A photo of the package is included with this release. The following information is printed on the back panel of each package (bag) as shown below. 2138XD PROD OF USA BEST BY AUG 01 2020 No illnesses have been reported to date and no other Pictsweet Farms products are impacted by this recall. Consumers who have purchased Pictsweet Farms 8 ounce Steam'ables Asparagus Spears with the code listed above may contact the Pictsweet consumer affairs line at 1-800-527-0986 from 9am to 5pm Central Standard Time, Monday – Friday. Do not consume affected product. Please dispose of the product or return it to the place of purchase for a full refund. Published 11/12/18
Duncan Hines Cake Mix Recall
Conagra Brands is collaborating with health officials in connection with a positive finding of Salmonella in a retail sample of Duncan Hines Classic White cake mix that may be linked to a Salmonella outbreak that is currently being investigated by CDC and FDA. While it has not been definitively concluded that this product is linked to the outbreak and the investigation is still ongoing, Conagra has decided to voluntarily recall the specific Duncan Hines variety identified (Classic White) and three other varieties (Classic Butter Golden, Signature Confetti and Classic Yellow) made during the same time period out of an abundance of caution. Five occurrences of illnesses due to Salmonella are being researched by CDC and FDA as part of this investigation. Salmonella is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis. Several of the individuals reported consuming a cake mix at some point prior to becoming ill, and some may have also consumed these products raw and not baked. Consumers are reminded not to consume any raw batter. Cake mixes and batter can be made with ingredients such as eggs or flour which can carry risks of bacteria that are rendered harmless by baking, frying or boiling. Consumers are reminded to wash their hands, work surfaces, and utensils thoroughly after contact with raw batter products, to follow baking instructions, and to never eat raw batter. Product information is listed below. No other Duncan Hines products or Conagra Brands' products are impacted by this recall. Consumers who have purchased these items are advised not to consume them and to return them to the store where originally purchased. Conagra Brands is cooperating with the FDA on this recall and is working with customers to ensure the packages are removed from store shelves and are no longer distributed. Consumers with questions should call the Conagra Consumer Care team at 1-888-299-7646, open 9 am through 5 pm EST, Monday through Friday or visit www.duncanhines.com. Published 11/6/18
Southwest Style Portabella Mushrooms
Renaissance Food Group has been notified that sweet corn used as an ingredient in some of their products is being voluntarily recalled by a supplier. According to the supplier, it has the potential to be contaminated with Listeria monocytogenes or Salmonella, both of which are microorganisms that can cause human illness.
| Product Name | UPC | Best if Sold By |
| Southwest Style Portabella Mushrooms | 8 2676671620 3 | 10/21/18 or prior |
They have not received any consumer complaints or reports of illnesses associated with the consumption of the recalled product to date. However, out of an abundance of caution, they are voluntarily recalling affected products containing the sweet corn ingredient. Consumers should not consume this product, it should be discarded or returned for a refund. Consumers with questions may contact Renaissance Food Group at (916) 638-8825. Published 10/16/18
Hostess Cookies ‘n Crème Brownies
Hostess Brands, LLC has become aware that the “Contains” statement on Hostess® Cookies ‘n Crème Brownies does not list “egg” as an allergen. Although the ingredient list on the packaging identifies “egg” as an ingredient, the “Contains” statement, which is designed to further alert consumers of allergens in the products, does not include “egg”. As a result and out of an abundance of caution, Hostess Brands is voluntarily recalling the following Cookies ‘n Crème Brownies:
| Product | Item UPC | Batch |
| Cookies ‘n Crème Brownie MP FSH | 20888109113579 | F052123000 |
| F061123000 | ||
| F071623000 | ||
| F072323000 | ||
| Cookies ‘n Crème Brownie MP FZN | 30888109213573 | F052123000 |
| Cookies ‘n Crème Brownie SS FSH | 20888109012025 | F052123000 |
| F061123000 | ||
| F071623000 | ||
| F072323000 | ||
| Cookies ‘n Crème Brownie SS FZN | 30888109022021 | F052123000 |
| F071623000 |
No other varieties of Hostess® Brownies are affected. No reports of injury or illness have been reported to date. Only those consumers who have an allergy or severe sensitivity to egg are potentially at risk of an allergic reaction if they consume these products. Anyone who has purchased an affected product and who has a sensitivity or allergy to eggs is encouraged to discard the affected product or return it to the place of purchase for a full refund. Consumers with questions may contact 1-800-483-7253 and also visit www.hostesscakes.com.
Brookshire’s Golden Hash Brown Patties Recall
The manufacturer of Brookshire’s Golden Hash Brown Patties, Lamb Weston, has issued a voluntary recall on this item due to the potential for these products to contain small fragments of hard, clear plastic. No other Lamb Weston products shipped to Brookshire Grocery Company are affected by this voluntary recall. No injuries have been reported to Lamb Weston related to its products that are associated with this recall.
| Product Name | UPC | Lot | Best By Date | Weight |
| Brookshire's Golden Hash Brown Patties | 0 92825 09630 6 | ALL | 090319 | 22.5 oz. (1 lb. 6.5 oz.) 638g |
Consumers who are impacted should contact Lamb Weston for product replacement or questions: (888) 912-9070 Monday-Friday 9:00 a.m. – 6:00 p.m. EDT. Published 9/21/18
Pepperidge Farm Goldfish Crackers
Pepperidge Farm has been notified by one of its ingredient suppliers that whey powder in a seasoning that is applied to four varieties of crackers has been the subject of a recall due to the potential presence of Salmonella. Pepperidge Farm initiated an investigation and, out of an abundance of caution, is voluntarily recalling four varieties of Goldfish crackers. No illnesses have been reported. No other Pepperidge Farm products are subject to this recall. The following four varieties with the indicated codes are subject to this recall:
- Flavor Blasted® Xtra Cheddar
- Flavor Blasted® Sour Cream & Onion
- Goldfish® Baked with Whole Grain Xtra Cheddar
- Goldfish® Mix Xtra Cheddar + Pretzel
Different packaging options are included in this recall. Consumers are encouraged to read the chart above. Consumers who have purchased these products should not eat them. Recalled product should be discarded or may be returned to the place of purchase for a full refund. Consumers with questions may visit www.pepperidgefarm.com/GoldfishUpdate or call Customer Service at 800-679-1791, 24 hours a day, for more information. Customer Service specialists are available M – F 9AM – 7PM EST. Published 07/24/18
Nabisco Ritz Sandwich Crackers and Ritz Bits
Mondelez Global LLC announced a voluntary recall of certain Ritz Cracker Sandwiches and Ritz Bits product. These products contain whey powder as an ingredient, which the whey powder supplier has recalled due to the potential presence of Salmonella. This recall is limited exclusively to the products listed below, available at retail stores nationwide. No other Mondelez Global LLC product is included in this recall. There have been no complaints of illness reported to Mondelez Global to date in connection with these products. The company is conducting this recall as a precaution, based on the ingredient supplier’s recall. Consumers who have these products should not eat them, and should discard any products they may have. Consumers can contact the company at 1-844-366 -1171, 24 hours a day to get more information about the recall, and Consumer Relations specialists are available Monday-Friday, 9am to 6pm EST. This recall is being conducted with the knowledge of the U.S. Food and Drug Administration. Published 07/23/18
Sparkling Ice Cherry Limeade Recall
Talking Rain® (the Company) is voluntarily recalling specific lot codes of bottles of its Sparkling Ice® Cherry Limeade beverage in response to a small number of customer complaints that reported an off-taste and off-odor of the affected product. The Company made the decision to recall the product out of an abundance of caution. No other Sparkling Ice products are affected by this recall. The Sparkling Ice Cherry Limeade product subject to recall was produced only at one of the Company’s facilities that make this product. The product comes in a 17 ounce clear plastic bottle under the name Sparkling Ice Cherry Limeade. The product is sold as a single item, as well as in multipacks of the affected product and in multipacks containing a variety of other unaffected flavors. The product subject to recall can be identified by the following information that is displayed on the neck of the bottle. All other products made by the Company are not part of this voluntary recall. Affected product labeling:
| Bottle Lot Code | Expiration Date on Bottle | Expiration Date | Bottle UPC | Case/Variety Pack UPCs |
| 8064-63 | 120518 | 12/05/2018 | 1657195084 | 16571950866 |
| 8065-63 | 120618 | 12/06/2018 | 16571950927 | |
| 8079-63 | 122018 | 12/20/2018 | 16571951283 | |
| 8080-63 | 122118 | 12/21/2018 | 16571950866 | |
| 8087-63 | 122818 | 12/28/2018 | 16571953126 | |
| 8088-63 | 122918 | 12/29/2018 | 16571953614 | |
| 8089-63 | 123018 | 12/30/2018 | ||
| 8112-63 | 012219 | 01/22/2019 | ||
| 8113-63 | 012319 | 01/23/2019 | ||
| 8114-63 | 012419 | 01/24/2019 | ||
| 8118-63 | 012819 | 01/28/2019 | ||
| 8119-63 | 012919 | 01/29/2019 | ||
| 8126-63 | 020619 | 02/06/2019 | ||
| 8131-63 | 021119 | 02/11/2019 | ||
| 8132-63 | 021219 | 02/12/2019 | ||
| 8144-63 | 022419 | 02/24/2019 | ||
| 8145-63 | 022519 | 02/25/2019 | ||
| 8146-63 | 022619 | 02/26/2019 | ||
| 8165-63 | 031419 | 03/14/2019 | ||
| 8166-63 | 031519 | 03/15/2019 | ||
| 8167-63 | 031619 | 03/16/2019 |
Talking Rain learned of the issue after a small number of customers brought their concerns to the company’s attention. Talking Rain is currently investigating the cause of the issue in collaboration with the U.S. Food and Drug Administration (FDA). Consumers who purchased the affected product are advised not to drink it and to call the Talking Rain customer center at 855-201-4333 to receive a coupon to replace their purchase. Published 06/30/18
KELLOGG’S HONEY SMACKS CEREAL RECALL
Kellogg Company today announced it is voluntarily recalling 15.3 oz. and 23 oz. packages of Kellogg's® Honey Smacks® cereal (with code dates listed below) because these products have the potential presence of Salmonella. No other Kellogg products are impacted by this recall. Kellogg launched an investigation with the third-party manufacturer who produces Honey Smacks immediately after being contacted by the Food & Drug Administration (FDA) and Centers for Disease Control (CDC) regarding reported illnesses. According to the CDC, use or consumption of products contaminated with Salmonella may result in serious illness. It can also produce serious and sometimes fatal infections in young children, frail or elderly people and others with weakened immune systems. Healthy individuals infected with Salmonella can experience fever, diarrhea, nausea, vomiting and abdominal pain. The illness usually lasts four to seven days, and most persons recover without treatment. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses. HOW TO IDENTIFY THE RECALLED PRODUCT The affected product includes the following varieties distributed across the United States as well as limited distribution in Costa Rica, Guatemala, Mexico, the Caribbean, Guam, Tahiti and Saipan. The BEST if Used By Date can be found on the top of the cereal box, and the UPC code can be found on the bottom of the box.
| Description (Retail) | UPC Code | Size | BEST if Used By Date |
| Honey Smacks (with limited distribution outside the U.S.) | 3800039103 | 15.3 oz | JUN 14, 2018 through JUN 14, 2019 |
| Honey Smacks | 3800014810 | 23 oz | JUN 14, 2018 through JUN 14, 2019 |
Kellogg is asking that people who purchased potentially affected product discard it and contact the company for a full refund. Consumers seeking more information, including images of these products, can visit www.kelloggs.com/honeysmacksrecall or call 1-800-962-1413 from Monday – Friday, from 9 a.m. to 6 p.m. ET as well as Saturday and Sunday from 10 a.m. – 4 p.m. ET. Receipt notice for purchases made since 6/1/2017, to run 4 times per customer over the next 30 days: Our records indicate you have purchased an item that may be affected by a recall due to the potential presence of Salmonella. “Kellogg’s Honey Smacks” with UPC 3800039103 and “Best if used by” dates between JUN 14, 2018 and JUN 14, 2019 are affected by this recall. Kellogg is asking that people who purchased potentially affected product discard it and contact the company for a full refund. Consumers seeking more information, including images of these products, can visit www.kelloggs.com/honeysmacksrecall or call 1-800-962-1413 from Monday – Friday, from 9 a.m. to 6 p.m. ET as well as Saturday and Sunday from 10 a.m. – 4 p.m. ET. Published 06/15/18
Stella Artois 11.2-ounce (330ml) bottles
Stella Artois has announced a voluntary recall of select packages containing 11.2-ounce (330ml) bottles of Stella Artois beer that may contain particles of glass. This recall applies to Stella Artois 6-packs, 12-packs, 18-packs, 24-packs, "Best of Belgium" multi-packs in the U.S. and Canada, and Stella Artois Légère 6-packs and 12-packs in the U.S. The recall does not affect other Stella Artois packaging formats, such as cans or draft or bottles of any other production codes. Consumers are advised not to consume or allow others to consume the potentially-affected product. SPECIFIC PRODUCTION CODES FOR POTENTIALLY-AFFECTED STELLA ARTOIS PACKAGES Consumers should follow these steps to assess whether their package of Stella Artois 11.2-ounce (330ml) bottles is potentially affected and as a result is subject to this voluntary recall. More detailed instructions can be found at https://stellaartois.expertinquiry.com . Consumers can also call their consumer hotline at 1-855-215-5824
- Does the package have one of the best before dates written below? (see attachment for further instructions).
- If so, does it have the corresponding Package Code?
- If so, does it have the corresponding Time Stamp?
| U.S. Stella Artois Production Codes | ||
| Best Before Date | Package Code | Time Stamp |
| 13/02/2018 | 49 | 02:00-06:00 |
| 13/02/2018 | 52 | 22:00-23:59 |
| 14/02/2018 | 52 | 00:00-02:00 |
| 14/02/2018 | 52 | 22:00-23:59 |
| 15/02/2018 | 52 | 00:00-05:00 |
| 19/02/2018 | 52, 55 | 02:00-12:00 |
| 20/02/2018 | 52,55 | 00:00-05:00 |
| 4/3/2018 | 55 | 21:00-23:59 |
| 5/3/2018 | 55 | 00:00-22:00 |
| 22/04/2018 | 55 | 22:00-23:59 |
| 23/04/2018 | 55 | 00:00-23:59 |
| 24/04/2018 | 55 | 00:00-03:00 |
| 7/5/2018 | 55 | 22:00-23:59 |
| 8/5/2018 | 55 | 00:00-11:00 |
| 6/6/2018 | 49 | 08:00-20:00 |
| 7/6/2018 | 49,52 | 22:00-23:59 |
| 8/6/2018 | 49 | 00:00-13:00 |
| 8/6/2018 | 52 | 00:00-07:00 |
| 29/08/2018 | 55 | 04:00-10:00 |
| 13/09/2018 | 55 | 23:00-23:59 |
| 14/09/2018 | 55 | 00:00-22:00 |
| 15/09/2018 | 55 | 06:00-23:59 |
| 16/09/2018 | 55 | 00:00-18:00 |
| 18/09/2018 | 55 | 01:00-08:00 |
| 21/09/2018 | 55 | 03:00-23:59 |
| 22/09/2018 | 55 | 00:00-03:00 |
| 23/09/2018 | 52 | 09:00-23:59 |
| 24/09/2018 | 52 | 00:00-12:00 |
| 25/09/2018 | 52 | 12:00-23:59 |
| 29/09/2018 | 52 | 04:00-14:00 |
| 6/10/2018 | 52 | 19:00-23:59 |
| 7/10/2018 | 52 | 00:00-02:00 |
| 9/10/2018 | 55 | 02:00-07:00 |
| 12/10/2018 | 49 | 05:00-10:00 |
| 27/10/2018 | 52 | 01:00-13:00 |
| 3/11/2018 | 55 | 18:00-23:59 |
| 3/11/2018 | 52 | 20:00-23:59 |
| 2/2/2019 | 55 | 18:00-23:59 |
| U.S. Stella Artois Légère Production Codes | ||
| Best Before Date | Package Code | Time Stamp |
| 6/10/2018 | 55 | 21:00-23:59 |
| 7/10/2018 | 55 | 00:00-02:00 |
| 12/10/2018 | 52 | 01:00-06:00 |
| Canada Stella Artois Production Codes | ||
| Best Before Date | Package Code | Time Stamp |
| 20/02/2018 | 49 | 00:00-06:00 |
| 25/04/2018 | 49 | 17:00-23:59 |
| 26/04/2018 | 49 | 00:00-04:00 |
| 20/5/2018 | 49 | 08:00-23:59 |
| 21/5/2018 | 49 | 00:00-01:00 |
| 7/6/2018 | 49,52 | 22:00-23:59 |
| 8/6/2018 | 49 | 00:00-13:00 |
| 8/6/2018 | 52 | 00:00-07:00 |
| 13/09/2018 | 55 | 21:00-23:59 |
| 14/09/2018 | 55 | 00:00-22:00 |
| 25/09/2018 | 52 | 12:00-23:59 |
| 26/09/2018 | 52 | 00:00-23:59 |
Published 04/02/18
Mary B’s Frozen Biscuit Dough Recall
Hom/Ade Foods, Inc is voluntarily recalling Mary B’s® brand biscuits due to potential contamination with Listeria monocytogenes. The problem was discovered in a product sampling conducted by an outside co-packer, who manufactured the product. Listeria monocytogenes is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women. The Mary B’s products affected are frozen bagged biscuits with the UPC codes listed below. All “Best If Used By” dates BEFORE September 23, 2018 and with the letter “M” immediately after the date are included in the recall. The code may be found on the back of the bag, lower right corner. No other code dates are affected.
| Item UPC | Description | Count per pkg |
| 2059300007 | MARY B’S JUMBO BUTTERMILK BISCUITS 35OZ | 10 / 3.5OZ |
| 2059300015 | MARY B’S BUTTERMILK BISCUITS 26.4OZ | 12 / 2.2OZ |
| 2059300020 | MARY B’S BUTTERMILK VALUE PACK BISCUITS 44OZ | 20 / 2.2OZ |
| 2059300018 | MARY B’S SOUTHERNMADE BISCUITS 26.4OZ | 12 / 2.2OZ |
| 2059300021 | MARY B’S SOUTHERNMADE VALUE PACK BISCUITS 44OZ | 20 / 2.2OZ |
| 2059300022 | MARY B’s BUTTERMILK TEA BISCUITS 24OZ | 24 / 1OZ |
| 2059300023 | MARY B’S BUTTERTASTE VALUE PACK BISCUITS 44OZ | 20 / 2.2OZ |
| 2059300028 | MARY B’S THIN BUTTERMILK BISCUITS 28.6OZ | 22 / 1.3OZ |
| 2059300033 | MARY B’S BUTTERMILK FAMILY PACK BISCUITS 60OZ | 30 / 2OZ |
| 2059300034 | MARY B’S SOUTHERNMADE FAMILY PACK BISCUITS 60OZ | 30 / 2OZ |
| 2059300035 | MARY B’S BUTTERTASTE FAMILY PACK BISCUITS 60OZ | 30 / 2OZ |
| 2059383000 | MARY B’S BUTTERMILK 0 TRANS FAT 220CT BULK BISCUITS | 220 / 2.2OZ |
| 2059383004 | MARY B’S BUTTERMILK BISCUITS 220CT BULK BISCUITS | 220 / 2.2OZ |
| 2059387000 | MARY B’S MADE WITH WHOLE GRAIN 220CT BULK BISCUITS | 220 / 2.2OZ |
| 3059320583 | MARY B’S 3.5 OZ JUMBO BUTTERMILK 144CT BULK BISCUITS | 144 / 3.5OZ |
While many items are affected by this recall, Brookshire Grocery Company only carries first 4 items on this list with the UPC’s 2059300007, 2059300015, 2059300020, 2059300023. Although these products are not ready-to-eat items and have baking instructions which, if followed, will reduce consumer risk, there remains some risk that the mishandling of this product prior to or without adequate baking may cause illness. There has been no illness to date associated with these products. Hom/Ade Foods Inc. has voluntarily recalled all affected products and is working with the FDA and supplier to fix the problem. Consumers are urged to return affected products to the place of purchase for a full refund. Customers or consumers with questions may call Hom/Ade Foods Inc. at 1-855-562-7773, Monday to Friday, between 8 a.m. and 5 p.m. Central Time. Published 01/09/18
United Pet Group Rawhide Chew Products Voluntary Recall United Pet Group, a division of Spectrum Brands, Inc. is voluntarily recalling multiple brands of packages of rawhide dog chew products that were distributed to retail outlets and sold online in the U.S. The recall involves the brands and products described below. The recall was initiated after United Pet Group identified that certain of its rawhide chew manufacturing facilities located in Mexico and Colombia, as well as one of its suppliers in Brazil, were using a quaternary ammonium compound mixture as a processing aid in the manufacturing of rawhide chews. The compound is an anti-microbial chemical that is approved for cleaning food processing equipment, but it has not been approved in the U.S. as a processing aid in the production of rawhide chews for dogs. United Pet Group received very limited reports of pet illness based on the volume of possibly affected rawhide chew products manufactured and distributed. The primary complaint received from consumers was that the affected product had an unpleasant odor. Diarrhea and vomiting were also reported. Exposure to quaternary ammonium compounds through direct ingestion may cause the following symptoms in dogs: reduced appetite, and gastric irritation including diarrhea and vomiting. These symptoms may require treatment by a veterinarian depending on severity. The affected product was distributed nationwide from United Pet Group’s Edwardsville, Illinois distribution facility and was delivered to consumers through various retail establishments including online outlets. All of the dog chew products included in the voluntary recall identify an expiration date ranging from 06/01/2019 through 05/31/2020 located on the back of the package. The products subject to the recall are described below.
| Product Brands | Product Names and Identifying Information |
| American Beefhide | United Pet Group is recalling certain packages of dog chews with the American Beefhide brand on the label. This recall is limited to dog chew products that contain rawhide. Only products with lot codes listed on the back of the package that start with AH and which list expiration dates from 06/01/2019 through 05/31/2020 are affected by this recall. This includes all package sizes and/or weights. The following contact information appears on the back of the package of the affected products: Manufactured by: Salix Animal Health, LLC Deerfield Beach, FL 33442 |
| Digest-eeze | United Pet Group is recalling certain packages of dog chews with the Digest-eeze brand on the label. This recall is limited to dog chew products that contain rawhide. Only products with lot codes listed on the back of the package that start with AH, AV, A, AI, AO, or AB, and which list expiration dates from 06/01/2019 through 05/31/2020 are affected by this recall. This includes all package sizes and/or weights. The following contact information appears on the back of the package of the affected products: Manufactured by: Salix Animal Health, LLC Deerfield Beach, FL 33442 |
| Healthy Hide (including Healthy Hide – Good -n- Fun and Healthy Hide – Good -n- Fit) | United Pet Group is recalling certain packages of dog chews with the Healthy Hide brand, Healthy Hide Good-n-Fit brand, and Healthy Hide Good-n-Fun brand on the label. |
This recall is limited to dog chew products that contain rawhide. Only products with lot codes listed on the back of the package that start with AH, AV, A, AI, AO, or AB and which list expiration dates from 06/01/2019 through 05/31/2020 are affected by this recall. This includes all package sizes and/or weights. The following contact information appears on the back of the package of the affected products: United Pet Group, a Division of Spectrum Brands, Inc. 3001 Commerce St. Blacksburg, VA 24060 1-800-645-5154 Consumers who have purchased the products described above are urged to dispose of the product or return it directly to United Pet Group or to the retail establishment where they initially purchased the product for full refund. If you have these products, please contact the United Pet Group consumer affairs team at 1-855-215-4962 between the hours of 8AM – 11PM EST for a refund. Consumers with questions may call the consumer affairs team at the number listed above. Full recall text and brand logos can be found at fda.gov/Safety/Recalls/ucm562701.htm Published 06/14/17
Gerber Cheese Ravioli Pasta Pick-Ups
 Gerber Products Company of Florham Park, New Jersey, is initiating a voluntary recall of Cheese Ravioli Gerber® Pasta Pick-Ups® because the egg allergen is missing from the "Contains" statement. The full ingredient list on the package does list "egg" as an ingredient; however, the "Contains" statement, designed to further alert parents to allergens in the recipe, did not include "egg" as is required. Only consumers who have an allergy or severe sensitivity to egg are at risk of serious allergic reaction if they consume this product. Cheese Ravioli Gerber® Pasta Pick-Ups® (UPC code: 150907) was distributed nationally through retail stores and ecommerce and is limited to the United States. This voluntary recall impacts all packages of the Cheese Ravioli variety of Gerber® Pasta Pick-Ups®. All other Gerber products, including other Gerber® Pasta Pick-Ups® varieties, are appropriately labeled. To date, no illness has been reported due to an allergic reaction to egg. This labeling oversight was brought to their attention as a result of a consumer contact. Following their own internal review, they confirmed egg was included in the ingredient list, but was not listed in the "Contains" statement. Gerber is in the process of updating its food package labels to make it easier for parents to identify foods that contain allergens such as egg, milk and wheat. On updated packages, this information can be found in the "Contains" statement as well as the ingredient list. Gerber regrets this oversight on their label. They encourage parents who have questions to contact them 24/7 at 1-800-510-7494. Published 03/08/17
Gerber Products Company of Florham Park, New Jersey, is initiating a voluntary recall of Cheese Ravioli Gerber® Pasta Pick-Ups® because the egg allergen is missing from the "Contains" statement. The full ingredient list on the package does list "egg" as an ingredient; however, the "Contains" statement, designed to further alert parents to allergens in the recipe, did not include "egg" as is required. Only consumers who have an allergy or severe sensitivity to egg are at risk of serious allergic reaction if they consume this product. Cheese Ravioli Gerber® Pasta Pick-Ups® (UPC code: 150907) was distributed nationally through retail stores and ecommerce and is limited to the United States. This voluntary recall impacts all packages of the Cheese Ravioli variety of Gerber® Pasta Pick-Ups®. All other Gerber products, including other Gerber® Pasta Pick-Ups® varieties, are appropriately labeled. To date, no illness has been reported due to an allergic reaction to egg. This labeling oversight was brought to their attention as a result of a consumer contact. Following their own internal review, they confirmed egg was included in the ingredient list, but was not listed in the "Contains" statement. Gerber is in the process of updating its food package labels to make it easier for parents to identify foods that contain allergens such as egg, milk and wheat. On updated packages, this information can be found in the "Contains" statement as well as the ingredient list. Gerber regrets this oversight on their label. They encourage parents who have questions to contact them 24/7 at 1-800-510-7494. Published 03/08/17
Sargento Expands Recall of Select Cheeses
Sargento Foods Inc. of Plymouth, Wis. is voluntarily expanding the recall of cheese supplied by Deutsch Kase Haus, LLC due to potential contamination with Listeria monocytogenes that originated from the supplier’s facility. There have been no confirmed illnesses. Out of concern for the health and well-being of Sargento consumers, the company has also terminated its relationship with Deutsch Kase Haus, which supplied Sargento with the affected Longhorn Colby cheese. Please note: both the UPC bar code number AND the associated “Sell By” date on your package of cheese must match this list to be part of the recall.
| UPC | Size | Description | Sell By Dates |
| 4610000105 | 8 oz. | Sargento Sliced Colby | 15MAY17F |
| 4610000107 | 8 oz. | Sargento Sliced Muenster | 05MAR17F 06MAR17F 16APR17F 17APR17F 15APR17F |
| 4610000122 | 7.5 oz. | Sargento Sliced Pepper Jack | 03MAY17B |
| 4610000279 | 6.67 oz. | Sargento Sliced Tomato & Basil Jack | 03MAR17B |
| 4610041018 | 8 oz. | Sargento Shredded Reduced Fat Colby-Jack | H07APR17 |
| 4610041105 | 8 oz. | Sargento Shredded Chef Blends 4 Cheese Pizzeria | H10APR17 |
| 4610040094 | 8 oz. | Sargento Artisan Blends Double Cheddar Shredded Cheese | H09JUN17 H08JUN17 |
| 4610000228 | 6.84 oz. | Ultra Thin Sliced Longhorn Colby | 02FEB17F 01MAR17B 16MAR17F 12APR17B 10MAY17B |
| 4610040041 | 8 oz. | Chef Blends Shredded Nacho & Taco | H04MAY17 S15MAY17 H01JUN17 H14JUN17 H12JUL17 |
| 4610000108 | 12 oz. | Sliced Pepper Jack | 02MAY17B 03MAY17B 11JUN17B 12JUN17B 09JUL17B 10JUL17B |
| 4610000109 | 12 oz. | Sliced Colby-Jack | 01MAY17B 11JUN17B |
| 4610040002 | 8 oz. | Chef Blends Shredded Taco | H11MAY17 H01JUN17 H14JUN17 F28JUN17 |
| 4610040014 | 8 oz. | Off the Block Fine Cut Shredded Colby-Jack | H06MAY17 F05JUL17 |
| 4610040076 | 8 oz. | Off the Block Fine Cut Shredded Cheddar Jack | H07MAY17 H08MAY17 H09MAY17 F05JUL17 |
Brookshire Grocery Company was notified by Sargento Foods Inc. that we received only one item from one affected lot:
| 4610040076 | 8 oz. | Off the Block Fine Cut Shredded Cheddar Jack | H07MAY17 |
Consumers can also check if they have recalled product by visiting info.sargento.com. This webpage will be updated with the latest information about the Feb. 10 recall and this voluntary expanded recall. Consumers can also call Sargento Consumer Affairs at 1-800-243-3737 between 9 a.m. and 4 p.m. (Central Time), or submit questions to the “Contact Us” page at sargento.com. Published 02/20/17
U.S. Smokeless Tobacco Company
U.S. Smokeless Tobacco Company (USSTC) is voluntarily recalling certain smokeless tobacco products, listed in the chart below, manufactured at USSTC’s facility in Franklin Park, IL. USSTC initiated the recall after receiving eight consumer complaints of foreign metal objects, including sharp metal objects, found in select cans. In each case, the object was visible to the consumer and there have been no reports of consumer injury. Complaints have been received from consumers in Indiana, Texas, North Carolina, Tennessee, Wisconsin and Ohio. The products at issue were manufactured solely in USSTC’s Franklin Park, IL facility and distributed nationally. The majority of USSTC’s cans are not affected, including Copenhagen Fine Cut in a fiberboard can, Copenhagen Long Cut in a fiberboard can and Copenhagen Long Cut Wintergreen in a plastic can. A consumer who has any of the products listed in the table below should not open or use the product. Consumers should contact USSTC at 1-866-201-9136 to return the product for a refund. The select cans subject to this recall:
| Cope Brand Products | |
| Long Cut Straight | This recall applies to lots with no printed code on the bottom of the can, or with codes that begin with the letters “F”, “R”, “K”, or “P”. |
| Copenhagen Brand Products | |
| Extra Long Cut Natural Long Cut Mint Long Cut Southern Blend Pouch Mint Pouch Wintergreen Long Cut (overseas military only) Fine Cut (overseas military only) Long Cut Straight (overseas military only) Long Cut Wintergreen (overseas military only) Pouch (overseas military only) Fine Cut Plastic Can (only available in Alaska and Hawaii) Long Cut Plastic Can (only available in Alaska and Hawaii) Pouch Plastic Can (only available in Alaska and Hawaii) | This recall applies to lots with no printed code on the bottom of the can, or with codes that begin with the letters “F”, “R”, “K”, or “P”. |
| Husky Brand Products | |
| Fine Cut Natural Long Cut Straight Long Cut Wintergreen | This recall applies to lots with no printed code on the bottom of the can, or with codes that begin with the letters “F”, “R”, “K”, or “P”. |
| Skoal Brand Products | |
| Bandit Mint Bandit Wintergreen Long Cut Apple Tobacco Blend Long Cut Berry Tobacco Blend Long Cut Cherry Long Cut Citrus Tobacco Blend Long Cut Classic Long Cut Peach Tobacco Blend Long Cut Spearmint Pouch Apple Tobacco Blend Pouch Berry Tobacco Blend Pouch Citrus Tobacco Blend Snus Mint Snus Smooth Mint Xtra Long Cut Mint Xtra Long Cut Rich Tobacco Blend Xtra Long Cut Wintergreen Xtra Pouch Crisp Tobacco Blend Xtra Pouch Mint Blend Xtra Pouch Rich Tobacco Blend Fine Cut Wintergreen (overseas military only) Long Cut Mint (overseas military only) Long Cut Straight (overseas military only) Long Cut Wintergreen (overseas military only) Pouch Mint (overseas military only) Pouches Wintergreen (overseas military only) | This recall applies to lots with no printed code on the bottom of the can, or with codes that begin with the letters “F”, “R”, “K”, or “P”. |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA Center for Tobacco Products safety reporting portal. This recall can be found on the FDA website. Published 02/01/17
Sierra Nevada Brewing Co.
Sierra Nevada Brewing Co. has announced a voluntary recall of select 12-ounce bottles that may contain a small glass packaging flaw. This recall comes after quality inspections at their Mills River, North Carolina, brewery detected a very limited number of bottles with a flaw that may result in loss of carbonation and a small piece of glass to break off and possibly fall into the bottle, causing a risk for injury. While they believe this concern impacts roughly 1 in every 10,000 (0.01%) of their bottles packaged during this time. To-date, they have not received any consumer reports of injuries resulting from the potentially affected bottles and are working with their suppliers to determine the root cause of the issue.
| Brand | Package Format | Production Date |
| Pale Ale | 12-ounce bottles in 6-, 12- and 24-pack (case) formats | 12/5/16 - 1/8/17 |
| Torpedo Extra IPA | 12-ounce bottles in 6-, 12- and 24-pack (case) formats | 12/5/16 - 1/13/17 |
| Tropical Torpedo | 12-ounce bottles in 6-pack format | 12/5/16 - 1/13/17 |
| Sidecar Orange Pale Ale | 12-ounce bottles in 6-, 12- and 24-pack (case) formats | 12/5/16 - 1/13/17 |
| Beer Camp Golden IPA | 12-ounce bottles in 6-, 12- and 24-pack (case) formats | 12/5/16 - 1/13/17 |
| Otra Vez | 12-ounce bottles in 6-pack format | 12/5/16 - 1/13/17 |
| Nooner | 12-ounce bottles in 6-pack format | 12/5/16 - 1/13/17 |
| Hop Hunter IPA | 12-ounce bottles in 6-, 12- and 24-pack (case) formats | 12/5/16 - 1/13/17 |
The specifics of the affected beer include those brands listed above with a package date that falls within the range AND a brewery code of “M” (Mills River) and not “C” (Chico), which is all information that can be found printed on the packaging and on the bottle. Consumers who have purchased beer within the scope of this recall will be eligible for full compensation of the purchase price and are advised not to drink it and to dispose of the beer. You may contact Sierra Nevada Brewing Co. at 800-596-7835 and leave your name, number and the nature of your question. This recall may be viewed in its entirety at sierranevada.com/qualitymatters Published on 01/24/17
Farm to Market Foods Recall
Farm to Market Foods is recalling the items listed below due to undeclared allergens. This recall has been initiated due to peanuts, a known allergen, to possibly be present in the product from an ingredient supplied by a secondary supplier and used in the manufacturing of the two listed side dishes. The ingredient being recalled is a Worcestershire Sauce where peanut was not declared on the packaging label. Use of, or consumption of, this product may induce a severe allergic reaction in some individuals.
| UPC # | Product Description |
| 061703795889 | Green Bean Casserole Side Dish |
| 061703795913 | Cajun Style Dirty Rice Side Dish |
| 061703795907 | Cajun Turkey Dinner containing the Cajun Style Dirty Rice Side Dish |
| 061703795908 | Hickory Smoke Half Spiral containing the Green Bean Casserole Side Dish |
| 061703795906 | Smoked Turkey Dinner containing the Green Bean Casserole Side Dish |
The problem was discovered on Dec. 22, 2016, when the firm was notified by their supplier that the Worcestershire Sauce used in the rice product was being recalled because the sauce may contain peanut. The establishment determined the scope of the recall by tracing the use of the recalled Worcestershire back to the October and November production dates. There have been no confirmed reports of adverse reactions due to consumption of these products. Anyone concerned about an injury or illness should contact a healthcare provider. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase. Published 12/26/16
Ron’s Home Style Foods, Inc.
Ron’s Home Style Foods, Inc. is recalling Tropical Fruit Supreme, Pineapple Nut Delight, and Pistachio Crème, because it has the potential to be contaminated with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e., infected aneurysms), endocarditis and arthritis. Tropical Fruit Supreme, Pineapple Nut Delight, and Pistachio Crème were distributed in Texas, Arkansas, and Oklahoma to foodservice distributors and retail supermarkets. Product is packaged in plastic containers with the following information on the label.
| Product | UPC | Size | Brand | Use By: |
| Tropical Fruit Supreme | 096938842027 | 5 lb. | Ron’s Home Style Foods | 12/27/16, 1/18/17, and 2/6/17 |
| Tropical Fruit Supreme | 046045022294 | 5 lb. | Golden Harvest | 12/27/16, 1/18/17, and 2/6/17 |
| Tropical Fruit Supreme | 096938841013 | 1 lb. | Texas Kitchen Salads | 12/27/16, 1/18/17, and 2/6/17 |
| Pineapple Nut Delight | 096938822029 | 5 lb. | Ron’s Home Style Foods | 12/27/16, 1/18/17, and 2/6/17 |
| Pineapple Nut Delight | 096938900086 | 12 oz. | Brookshire’s | /27/16, 1/18/17, and 2/6/17 |
| Pistachio Crème | 096938832028 | 5 lb. | Ron’s Home Style Foods | 12/27/16, 1/2/17, 1/18/17, and 2/6/17 |
| Pistachio Crème | 096938831014 | 1 lb. | Texas Kitchen Salads | 12/27/16, 1/2/17, 1/18/17, and 2/6/17 |
| Pistachio Crème | 096938900079 | 12 oz. | Brookshire’s | 12/27/16, 1/2/17, 1/18/17, and 2/6/17 |
No illnesses have been reported to date. The recall is due to a recall of an ingredient used in manufacturing these products. Valley Milk Products reported that certain lots of dry milk they manufactured have the potential to be contaminated with Salmonella. While Pistachio Crème does not contain the ingredient, it is made on the same production line as Tropical Fruit Supreme and Pineapple Nut Delight therefore we are recalling it as well. Consumers who have purchased the above products are urged not to consume the product and to return it to the place of purchase for a full refund. Consumers with questions may contact the company at 1.800.856.3131, M-F 8am-4pm CST. Published 12/16/16
Sabra Dipping Co., LLC
Colonial Heights, VA - Sabra Dipping Co., LLC is voluntarily recalling certain hummus products made prior to 11/08/16 due to concerns over Listeria monocytogenes, which was identified at the manufacturing facility but not in tested finished product. The recall includes the products listed below; these were distributed to retail outlets, including food service accounts and supermarkets, in the U.S. and Canada. Listeria monocytogenes is an organism, which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Healthy individuals may suffer only short-term symptoms such as high fever, severe headaches, stiffness, nausea, abdominal pain and diarrhea. Listeria infection can cause miscarriages and stillbirths among pregnant women. The company is issuing this recall out of an abundance of caution. Consumers with any product with a “Best Before” date up through 01/23/17 are urged to discard it. Consumers can find code and “Best Before” date on the lid of each package.
| UPC | SKU | Item |
|---|---|---|
| 40822014700 | 300051 | Sabra Hummus Caramelized Onion 10OZ |
| 40822000017 | 300066 | Sabra Hummus Classic 7OZ |
| 40822011143 | 300067 | Sabra Hummus Classic 10OZ |
| 40822017497 | 300070 | Sabra Hummus Classic 17OZ |
| 40822014687 | 300074 | Sabra Hummus Classic 30OZ |
| 40822431156 | 300076 | Sabra Hummus Classic 5LB - 6ct |
| 40822011112 | 300079 | Sabra Hummus Classic 2OZ - 48ct: 3 x (16 x 2oz) |
| 40822011952 | 300080 | Sabra Hummus Classic with pretzels 4.56OZ |
| 40822011235 | 300094 | Sabra Hummus Garlic 7OZ |
| 40822011242 | 300095 | Sabra Hummus Garlic 10OZ |
| 40822017510 | 300097 | Sabra Hummus Garlic 17OZ |
| 40822012256 | 300099 | Sabra Hummus Garlic 32OZ |
| 40822301121 | 300100 | Sabra Hummus Garlic 30OZ |
| 40822011990 | 300104 | Sabra Hummus Garlic with pretzels 4.56OZ |
| 40822011921 | 300106 | Sabra Hummus Jalapeno 10OZ |
| 40822011341 | 300117 | Sabra Hummus Olive 10OZ |
| 40822011747 | 300132 | Sabra Hummus Pine Nut 10OZ |
| 40822127530 | 300134 | Sabra Hummus Pine Nut 7OZ |
| 40822990011 | 300136 | Sabra Hummus Pine Nut 17OZ |
| 40822012157 | 300139 | Sabra Hummus Pine Nut 32OZ |
| 40822012430 | 300142 | Sabra Hummus Red Pepper 7OZ |
| 40822011549 | 300143 | Sabra Hummus Red Pepper 10OZ |
| 40822017503 | 300146 | Sabra Hummus Red Pepper 17OZ |
| 40822328647 | 300148 | Sabra Hummus Red Pepper 32OZ |
| 40822301114 | 300150 | Sabra Hummus Red Pepper 30OZ |
| 40822434553 | 300151 | Sabra Hummus Red Pepper 5LB - 6ct |
| 40822011969 | 300153 | Sabra Hummus Red Pepper with pretzels 4.56OZ |
| 40822011433 | 300158 | Sabra Hummus Supremely Spicy 7OZ |
| 40822011440 | 300159 | Sabra Hummus Supremely Spicy 10OZ |
| 40822017558 | 300161 | Sabra Hummus Supremely Spicy 17OZ |
| 40822027540 | 300164 | Sabra Hummus Spinach & Artichoke 10OZ |
| 40822014731 | 300166 | Sabra Hummus Sun Dried Tomato 10OZ |
| 40822027700 | 300266 | Sabra Hummus Spinach & Artichoke 32OZ |
| 40822027588 | 300298 | Sabra Hummus Spinach & Artichoke 17OZ |
| 40822990011 | 300501 | Sabra Hummus Pine Nut 17OZ - 6ct |
| 40822017503 | 300502 | Sabra Hummus Red Pepper 17OZ - 6ct |
| 40822020114 | 300593 | Sabra Hummus Basil-Pesto 10OZ |
| 40822330466 | 300736 | Sabra Hummus Tuscan Herb Garden 32OZ |
| 40822342049 | 301216 | Sabra Hummus Classic 32OZ |
| 40822342131 | 301271 | Sabra Hummus Classic with pretzels 4.56OZ - 8ct |
| 40822342209 | 301283 | Sabra Hummus Garlic 23.5OZ |
| 40822017497 | 301290 | Sabra Hummus Classic 17OZ |
| 40822342506 | 301430 | Sabra Hummus Bold & Spicy with tortilla chips 4.56OZ |
| 40822017510 | 301480 | Sabra Hummus Garlic 17OZ - 6ct |
| 40822342728 | 301481 | Sabra Hummus Classic 2OZ - 6 x 2oz (12 x 6pks) |
| 40822011648 | 301483 | Sabra Hummus Lemon 10OZ |
| 40822342735 | 301484 | Sabra Hummus Red Pepper 2OZ - 6 x 2oz (12 x 6pks) |
| 40822330381 | 301485 | Sabra Hummus Tuscan Herb Garden 17OZ |
| 40822010078 | 301511 | Sabra Hummus Classic 2OZ - 16 x 2oz - 12 ct |
| 40822010047 | 301512 | Sabra Hummus Classic 2OZ - 12 x 2oz - 12 ct |
| 40822342988 | 301566 | Sabra Hummus SF Rosemary/Sea Salt 10OZ |
| 40822343145 | 301585 | Sabra Spreads Spicy Chili 8.5OZ - 8ct |
| 40822343138 | 301586 | Sabra Spreads Garlic Herb 8.5OZ - 8ct |
| 40822343121 | 301587 | Sabra Spreads Honey Mustard 8.5OZ - 8ct |
| 40822343114 | 301588 | Sabra Spreads Salt & Pepper 8.5OZ - 8ct |
| 40822343671 | 301640 | Sabra Hummus Taco 10OZ |
| 40822344043 | 301705 | Sabra Hummus 3 Pepper Chili 10OZ |
No other Sabra products are affected. In particular, Sabra products not included in the recall are: Sabra Organic Hummus, Sabra Salsa, Sabra Guacamole and Sabra Greek Yogurt Dips. Consumers can contact Sabra Consumer Relations at 1-866-265-6761 for additional information from 9am to 8PM eastern time. For product reimbursement, consumers can contact Sabra. The company has subsequently taken steps to correct this matter. The recall is being conducted with the knowledge of the U.S. Food and Drug Administration. Published 11/21/16
Back to Nature Foods
Back to Nature Foods, LLC has issued a voluntarily recall of four products because those products contain chocolate purchased from a third party supplier which was found to contain undeclared milk that is not listed as an ingredient on the label. Products affected are:
| Back to Nature Products | UPC | Best By Date |
| Chocolate Delight Granola 11oz | 19898-01201 | 12/25/16 - 7/27/17 |
| Dark Chocolate Coconut Granola 11oz | 19898-01225 | 5/9/17 - 8/14/17 |
| Chocolate Chunk Cookies 9.5oz | 19898-01100 | 5/8/17 – 5/12/17 |
| Mini Chocolate Chunk Cookies 6 x 1.25oz | 19898-01114 (Tray) 19898-01115 (Pouch) | 5/1/17 |
| Mini Chocolate Chunk Cookies 100 x 1.25oz | 19898-01148 (Case) 19898-01115 (Pouch) | 5/1/17 |
| Mini Chocolate Chunk Cookies 9 x 2.5oz | 19898-01144 (Caddy) 19898-01143 (Pouch) | 5/1/17 |
Persons who have an allergy or severe sensitivity to milk run the risk of a serious or life-threatening allergic reaction if they consume this product. Best by date codes can be found on the top of the bottom of the Granola packaging, the top of the Chocolate Chunk 9.5 oz carton, and on the back of the Mini Chocolate Chunk Tray or individual package. Consumers who have purchased the Back to Nature Chocolate Chunk Cookies, Mini Chocolate Chunk Cookies, Chocolate Delight Granola, or Dark Chocolate Coconut Granola with the UPC numbers and dates noted, and have an allergy to milk, should destroy the product they have or are urged to return the product to the place of purchase for product replacement or refund. Consumers with questions may call Back to Nature’s Consumer Relations Center at (844) 275-5845. The center is open Monday through Friday from 9am-5pm Eastern. Consumers also may contact the center via e-mail by visiting the Contact Us page at backtonaturefoods.com/contact-us/ for a replacement coupon. No other Back to Nature brand products are included in this recall. Published 10/28/16
Blue Bell Cookie Dough Ice Cream Recall
Blue Bell Ice Cream is voluntarily recalling all products that were made with a cookie dough ingredient supplied by a third party supplier Aspen Hills, Inc., due to the potential for them to contain Listeria monocytogenes. This recall includes the following products in half gallons and pints, Blue Bell Chocolate Chip Cookie Dough and Blue Bell Cookie Two Step sold to retail outlets, and three gallon flavors sold to food service accounts including Blue Bell Blue Monster, Blue Bell Chocolate Chip Cookie, and Blue Bell Krazy Kookie Dough. These products were produced from 02/02/16 - 09/07/16. For more information, consumers with questions may call (979) 836-7977, Monday – Friday 8am–5pm CST. The full Blue Bell press release regarding this recall is available here. Published 10/11/16
Food Club Iodized Salt
A voluntary recall has been announced for Food Club Iodized Salt, UPC 00036800510166 with the lot code "F 6TA30" and date "06/2021" printed on the bottom. This item may contain plain salt rather than iodized salt. Please dispose of this product or return it for a refund. Published 08/23/16
